Isolation and feeder-free primary culture of four cell types from a single human skin sample
- PMID: 35199036
- PMCID: PMC8844903
- DOI: 10.1016/j.xpro.2022.101172
Isolation and feeder-free primary culture of four cell types from a single human skin sample
Abstract
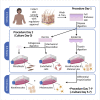
Four types of primary cells-dermal fibroblasts, dermal microvascular endothelial cells, epidermal keratinocytes, and epidermal melanocytes-can be isolated simultaneously from a single human skin sample, without the use of xenogeneic murine feeder cells. This protocol describes the procedures for isolation of these cells from adult full-thickness skin obtained from surgical discard tissue. The cells isolated using this protocol contain stem cell populations and are competent to form functional skin tissue in three-dimensional reconstructed skin models. For complete details on the use and execution of this profile, please refer to Supp et al. (2002), Boyce et al. (2015), Boyce et al. (2017a), Boyce et al. (2017b), and Supp et al. (2019).
Keywords: Cell Biology; Cell culture; Cell isolation; Stem Cells; Tissue Engineering.
© 2022 The Author(s).
Conflict of interest statement
The authors have no competing interests to declare related to the content of this manuscript.
Figures







References
-
- Bourland J., Mayrand D., Tremblay N., Moulin V.J., Fradette J., Auger F.A. Isolation and culture of human dermal microvascular endothelial cells. Methods Mol. Biol. 2019;1993:79–90. - PubMed
-
- Boyce S.T. Methods for the serum-free culture of keratinocytes and transplantation of collagen-gag-based skin substitutes. Methods Mol. Med. 1999;18:365–389. - PubMed
-
- Boyce S.T., Ham R.G. Cultivation, frozen storage, and clonal growth of normal human epidermal keratinocytes in serum-free media. J. Tiss. Cult. Meth. 1985;9:83–93.
-
- Boyce S.T., Kagan R.J., Greenhalgh D.G., Warner P., Yakuboff K.P., Palmieri T., Warden G.D. Cultured skin substitutes reduce requirements for harvesting of skin autograft for closure of excised, full-thickness burns. J. Trauma. 2006;60:821–829. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

