Peripheral neuropathy in the pre-diabetic state of the type 2 diabetes mouse model (TSOD mice) involves TRPV1 expression in dorsal root ganglions
- PMID: 35199097
- PMCID: PMC8850332
- DOI: 10.1016/j.ibneur.2022.02.001
Peripheral neuropathy in the pre-diabetic state of the type 2 diabetes mouse model (TSOD mice) involves TRPV1 expression in dorsal root ganglions
Abstract
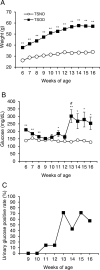
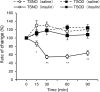
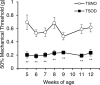
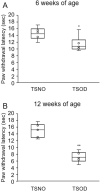
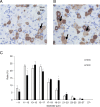
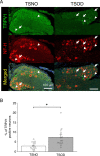
Peripheral neuropathy, which is a complication of diabetes mellitus (DM), is thought to occur in the pre-DM state, being known as impaired glucose tolerance (IGT) neuropathy, although its pathogenesis is unknown. Since it is reversible, an effective treatment at the pre-DM stage could stop the progression of peripheral neuropathy and improve patients' quality of life and reduce medical costs. We investigated the hypersensitivity to mechanical and thermal stimuli during the pre-DM state in Tsumura Suzuki Obese Diabetes (TSOD) mice, a type 2 DM mouse model. The expression pattern of the Transient Receptor Potential Vanilloid 1 (TRPV1)-positive cells in the dorsal root ganglia (DRG) was examined in TSOD mice, which showed a pre-DM state at 5-12 weeks of age and decreased mechanical and thermal nociceptive thresholds. Additionally, the size of TRPV1-positive cells in TSOD mice increased compared with that in non-diabetic controls (Tsumura Suzuki Non-Obesity; TSNO). Furthermore, the expression of TRPV1 on myelinated nerve fibers (neurofilament heavy-positive cells) had significantly increased. Thus, TSOD mice in the pre-DM state at 5-12 weeks of age could be a useful animal model of IGT neuropathy. We also hypothesized that the development of IGT neuropathy may involve a switch in TRPV1 expression from small, unmyelinated neurons to large, myelinated neurons in the DRG.
Keywords: ANOVA, Analysis of variance; DM, diabetes mellitus; DRG, dorsal root ganglion; Diabetes mellitus; FITC, fluorescein isothiocyanate; HFD, High-Fat Diet; IGT, impaired glucose tolerance; ITT, insulin tolerance test; Impaired glucose tolerance; LPA, Lysophosphatidic Acid; Mechanical allodynia; NF-H, neurofilament heavy; STZ, streptozotocin; TRITC, tetramethylrhodamine; TRPV1, Transient Receptor Potential Vanilloid 1; TSNO, Tsumura Suzuki Non-Obesity; TSOD, Tsumura Suzuki Obese Diabetes; Thermal hypersensitivity; ir, immunoreactive; tHODE, total Hydroxyoctadecadienoic Acid.
© 2022 The Authors.
Conflict of interest statement
None.
Figures
References
-
- Drel V.R., Pacher P., Stavniichuk R., Xu W., Zhang J., Kuchmerovska T.M., Slusher B., Obrosova I.G. Poly(ADP-ribose)polymerase inhibition counteracts renal hypertrophy and multiple manifestations of peripheral neuropathy in diabetic Akita mice. Int. J. Mol. Med. 2011;28(4):629–635. doi: 10.3892/ijmm.2011.709. - DOI - PMC - PubMed
-
- Dyck P.J., Kratz K.M., Karnes J.L., Litchy W.J., Klein R., Pach J.M., Wilson D.M., O’Brien P.C., Melton L.J., 3rd, Service F.J. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: The Rochester Diabetic Neuropathy Study. Neurology. 1993;43(4):817–824. doi: 10.1212/wnl.43.4.817. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
