Impact of polymorphisms of pharmacokinetics-related genes and the inflammatory response on the metabolism of voriconazole
- PMID: 35199485
- PMCID: PMC8866912
- DOI: 10.1002/prp2.935
Impact of polymorphisms of pharmacokinetics-related genes and the inflammatory response on the metabolism of voriconazole
Abstract
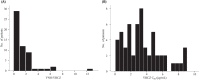
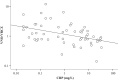
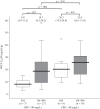
The effects of inflammatory responses and polymorphisms of the genes encoding cytochrome P450 (CYP) (CYP2C19 and CYP3A5), flavin-containing monooxygenase 3 (FMO3), pregnane X receptor (NR1I2), constitutive androstane receptor (NR1I3), and CYP oxidoreductase (POR) on the ratio of voriconazole (VRCZ) N-oxide to VRCZ (VNO/VRCZ) and steady-state trough concentrations (C0h ) of VRCZ were investigated. A total of 56 blood samples were collected from 36 Japanese patients. Results of multiple linear regression analyses demonstrated that the presence of the extensive metabolizer CYP2C19 genotype, the dose per administration, and the presence of the NR1I2 rs3814057 C/C genotype were independent factors influencing the VNO/VRCZ ratio in patients with CRP levels of less than 40 mg/L (standardized regression coefficients (SRC) = 0.448, -0.301, and 0.390, respectively; all p < .05). With regard to the concentration of VRCZ itself, in addition to the above factors, the presence of the NR1I2 rs7643645 G/G and rs3814055 T/T genotypes were found to be independent factors influencing the VRCZ C0h in these patients (SRC = -0.430, 0.424, -0.326, 0.406 and -0.455, respectively; all p < .05). On the contrary, in patients with CRP levels of at least 40 mg/L, no independent factors were found to affect VNO/VRCZ and VRCZ C0h . Inflammatory responses, and CYP2C19 and NR1I2 polymorphisms may be useful information for the individualization of VRCZ dosages.
Keywords: CRP; CYP2C19; NR1I2 polymorphism; voriconazole; voriconazole N-oxide.
© 2022 The Authors. Pharmacology Research & Perspectives published by John Wiley & Sons Ltd, British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics.
Figures

References
-
- Scott LJ, Simpson D. Voriconazole: a review of its use in the management of invasive fungal infections. Drugs. 2007;67(2):269‐298. - PubMed
-
- Hicheri Y, Cook G, Cordonnier C. Antifungal prophylaxis in haematology patients: the role of voriconazole. Clin Microbiol Infect. 2012;18(Suppl 2):1‐15. - PubMed
-
- Theuretzbacher U, Ihle F, Derendorf H. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet. 2006;45(7):649‐663. - PubMed
-
- Elewa H, El‐Mekaty E, El‐Bardissy A, et al. Therapeutic drug monitoring of voriconazole in the management of invasive fungal infections: a critical review. Clin Pharmacokinet. 2015;54(12):1223‐1235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

