Tailored Pyridoxal Probes Unravel Novel Cofactor-Dependent Targets and Antibiotic Hits in Critical Bacterial Pathogens
- PMID: 35199904
- PMCID: PMC9321722
- DOI: 10.1002/anie.202117724
Tailored Pyridoxal Probes Unravel Novel Cofactor-Dependent Targets and Antibiotic Hits in Critical Bacterial Pathogens
Abstract
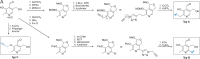
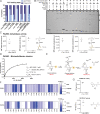
Unprecedented bacterial targets are urgently needed to overcome the resistance crisis. Herein we systematically mine pyridoxal phosphate-dependent enzymes (PLP-DEs) in bacteria to focus on a target class which is involved in crucial metabolic processes. For this, we tailored eight pyridoxal (PL) probes bearing modifications at various positions. Overall, the probes exceeded the performance of a previous generation and provided a detailed map of PLP-DEs in clinically relevant pathogens including challenging Gram-negative strains. Putative PLP-DEs with unknown function were exemplarily characterized via in-depth enzymatic assays. Finally, we screened a panel of PLP binders for antibiotic activity and unravelled the targets of hit molecules. Here, an uncharacterized enzyme, essential for bacterial growth, was assigned as PLP-dependent cysteine desulfurase and confirmed to be inhibited by the marketed drug phenelzine. Our approach provides a basis for deciphering novel PLP-DEs as essential antibiotic targets along with corresponding ways to decipher small molecule inhibitors.
Keywords: Antibiotic Compound Screening; Cofactors; Enzyme Characterisation; Proteomics; Pyridoxal Phosphate-Dependent Enzymes.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Mining the cellular inventory of pyridoxal phosphate-dependent enzymes with functionalized cofactor mimics.Nat Chem. 2018 Dec;10(12):1234-1245. doi: 10.1038/s41557-018-0144-2. Epub 2018 Oct 8. Nat Chem. 2018. PMID: 30297752 Free PMC article.
-
Bioisosteric replacement of pyridoxal-5'-phosphate to pyridoxal-5'-tetrazole targeting Bacillus subtilis GabR.Protein Sci. 2025 Jan;34(1):e70014. doi: 10.1002/pro.70014. Protein Sci. 2025. PMID: 39720892 Free PMC article.
-
Pyridoxal Reductase, PdxI, Is Critical for Salvage of Pyridoxal in Escherichia coli.J Bacteriol. 2020 May 27;202(12):e00056-20. doi: 10.1128/JB.00056-20. Print 2020 May 27. J Bacteriol. 2020. PMID: 32253339 Free PMC article.
-
Biomedical aspects of pyridoxal 5'-phosphate availability.Front Biosci (Elite Ed). 2012 Jan 1;4(3):897-913. doi: 10.2741/E428. Front Biosci (Elite Ed). 2012. PMID: 22201923 Review.
-
A genomic overview of pyridoxal-phosphate-dependent enzymes.EMBO Rep. 2003 Sep;4(9):850-4. doi: 10.1038/sj.embor.embor914. EMBO Rep. 2003. PMID: 12949584 Free PMC article. Review.
Cited by
-
Activity-based protein profiling in microbes and the gut microbiome.Curr Opin Chem Biol. 2023 Oct;76:102351. doi: 10.1016/j.cbpa.2023.102351. Epub 2023 Jul 8. Curr Opin Chem Biol. 2023. PMID: 37429085 Free PMC article. Review.
-
Translation Fidelity and Respiration Deficits in CLPP-Deficient Tissues: Mechanistic Insights from Mitochondrial Complexome Profiling.Int J Mol Sci. 2023 Dec 15;24(24):17503. doi: 10.3390/ijms242417503. Int J Mol Sci. 2023. PMID: 38139332 Free PMC article.
-
Chemoproteomic Approaches for Unraveling Prokaryotic Biology.Isr J Chem. 2023 Mar;63(3-4):e202200076. doi: 10.1002/ijch.202200076. Epub 2023 Feb 28. Isr J Chem. 2023. PMID: 37842282 Free PMC article.
-
Exploring antibiotic resistance with chemical tools.Chem Commun (Camb). 2023 May 18;59(41):6148-6158. doi: 10.1039/d3cc00759f. Chem Commun (Camb). 2023. PMID: 37039397 Free PMC article. Review.
-
Advancing Covalent Ligand and Drug Discovery beyond Cysteine.Chem Rev. 2025 Jul 23;125(14):6653-6684. doi: 10.1021/acs.chemrev.5c00001. Epub 2025 May 22. Chem Rev. 2025. PMID: 40404146 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

