Scavenging mitochondrial hydrogen peroxide by peroxiredoxin 3 overexpression attenuates contractile dysfunction and muscle atrophy in a murine model of accelerated sarcopenia
- PMID: 35199907
- PMCID: PMC8920438
- DOI: 10.1111/acel.13569
Scavenging mitochondrial hydrogen peroxide by peroxiredoxin 3 overexpression attenuates contractile dysfunction and muscle atrophy in a murine model of accelerated sarcopenia
Abstract
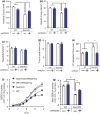
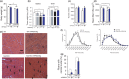
Age-related muscle atrophy and weakness, or sarcopenia, are significant contributors to compromised health and quality of life in the elderly. While the mechanisms driving this pathology are not fully defined, reactive oxygen species, neuromuscular junction (NMJ) disruption, and loss of innervation are important risk factors. The goal of this study is to determine the impact of mitochondrial hydrogen peroxide on neurogenic atrophy and contractile dysfunction. Mice with muscle-specific overexpression of the mitochondrial H2 O2 scavenger peroxiredoxin3 (mPRDX3) were crossed to Sod1KO mice, an established mouse model of sarcopenia, to determine whether reduced mitochondrial H2 O2 can prevent or delay the redox-dependent sarcopenia. Basal rates of H2 O2 generation were elevated in isolated muscle mitochondria from Sod1KO, but normalized by mPRDX3 overexpression. The mPRDX3 overexpression prevented the declines in maximum mitochondrial oxygen consumption rate and calcium retention capacity in Sod1KO. Muscle atrophy in Sod1KO was mitigated by ~20% by mPRDX3 overexpression, which was associated with an increase in myofiber cross-sectional area. With direct muscle stimulation, maximum isometric specific force was reduced by ~20% in Sod1KO mice, and mPRDX3 overexpression preserved specific force at wild-type levels. The force deficit with nerve stimulation was exacerbated in Sod1KO compared to direct muscle stimulation, suggesting NMJ disruption in Sod1KO. Notably, this defect was not resolved by overexpression of mPRDX3. Our findings demonstrate that muscle-specific PRDX3 overexpression reduces mitochondrial H2 O2 generation, improves mitochondrial function, and mitigates loss of muscle quantity and quality, despite persisting NMJ impairment in a murine model of redox-dependent sarcopenia.
Keywords: aging; hydrogen peroxide; mitochondria; peroxiredoxin3; sarcopenia.
© 2022 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
Modulation of sarcopenia phenotypes by glutathione peroxidase 4 overexpression in mice.J Physiol. 2023 Dec;601(23):5277-5293. doi: 10.1113/JP285259. Epub 2023 Oct 25. J Physiol. 2023. PMID: 37878529 Free PMC article.
-
Muscle mitochondrial catalase expression prevents neuromuscular junction disruption, atrophy, and weakness in a mouse model of accelerated sarcopenia.J Cachexia Sarcopenia Muscle. 2021 Dec;12(6):1582-1596. doi: 10.1002/jcsm.12768. Epub 2021 Sep 24. J Cachexia Sarcopenia Muscle. 2021. PMID: 34559475 Free PMC article.
-
Cancer cachexia in a mouse model of oxidative stress.J Cachexia Sarcopenia Muscle. 2020 Dec;11(6):1688-1704. doi: 10.1002/jcsm.12615. Epub 2020 Sep 12. J Cachexia Sarcopenia Muscle. 2020. PMID: 32918528 Free PMC article.
-
Accelerated sarcopenia in Cu/Zn superoxide dismutase knockout mice.Free Radic Biol Med. 2019 Feb 20;132:19-23. doi: 10.1016/j.freeradbiomed.2018.06.032. Epub 2018 Jul 2. Free Radic Biol Med. 2019. PMID: 30670156 Free PMC article. Review.
-
Reactive oxygen species in the pathogenesis of sarcopenia.Free Radic Biol Med. 2025 Feb 1;227:446-458. doi: 10.1016/j.freeradbiomed.2024.11.046. Epub 2024 Nov 28. Free Radic Biol Med. 2025. PMID: 39613046 Free PMC article. Review.
Cited by
-
Senolytic treatment does not mitigate oxidative stress-induced muscle atrophy but improves muscle force generation in CuZn superoxide dismutase knockout mice.Geroscience. 2024 Jun;46(3):3219-3233. doi: 10.1007/s11357-024-01070-x. Epub 2024 Jan 18. Geroscience. 2024. PMID: 38233728 Free PMC article.
-
Modulation of sarcopenia phenotypes by glutathione peroxidase 4 overexpression in mice.J Physiol. 2023 Dec;601(23):5277-5293. doi: 10.1113/JP285259. Epub 2023 Oct 25. J Physiol. 2023. PMID: 37878529 Free PMC article.
-
Aging-associated Aberrant Mitochondrial Redox Signaling, Physical Activity, and Sarcopenia.Curr Aging Sci. 2025;18(2):120-131. doi: 10.2174/0118746098315667240606052523. Curr Aging Sci. 2025. PMID: 38920079 Review.
-
The Complex Interplay between Mitochondria, ROS and Entire Cellular Metabolism.Antioxidants (Basel). 2022 Oct 8;11(10):1995. doi: 10.3390/antiox11101995. Antioxidants (Basel). 2022. PMID: 36290718 Free PMC article. Review.
-
Histological Methods to Assess Skeletal Muscle Degeneration and Regeneration in Duchenne Muscular Dystrophy.Int J Mol Sci. 2022 Dec 16;23(24):16080. doi: 10.3390/ijms232416080. Int J Mol Sci. 2022. PMID: 36555721 Free PMC article. Review.
References
-
- Ahn, B. , Ranjit, R. , Premkumar, P. , Pharaoh, G. , Piekarz, K. M. , Matsuzaki, S. , Claflin, D. R. , Riddle, K. , Judge, J. , Bhaskaran, S. , Satara Natarajan, K. , Barboza, E. , Wronowski, B. , Kinter, M. , Humphries, K. M. , Griffin, T. M. , Freeman, W. M. , Richardson, A. , Brooks, S. V. , & Van Remmen, H. (2019). Mitochondrial oxidative stress impairs contractile function but paradoxically increases muscle mass via fibre branching. Journal of Cachexia, Sarcopenia and Muscle, 10(2), 411–428. 10.1002/jcsm.12375 - DOI - PMC - PubMed
-
- Ahn, B. , Smith, N. , Saunders, D. , Ranjit, R. , Kneis, P. , Towner, R. A. , & Van Remmen, H. (2019). Using MRI to measure in vivo free radical production and perfusion dynamics in a mouse model of elevated oxidative stress and neurogenic atrophy. Redox Biology, 26, 101308. 10.1016/j.redox.2019.101308 - DOI - PMC - PubMed
-
- Andersen, J. L. (2003). Muscle fibre type adaptation in the elderly human muscle. Scandinavian Journal of Medicine and Science in Sports, 13, 40–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

