Switchable Regioselective 6- endo or 5- exo Radical Cyclization via Photoredox Catalysis
- PMID: 35200024
- PMCID: PMC8925928
- DOI: 10.1021/jacs.2c00192
Switchable Regioselective 6- endo or 5- exo Radical Cyclization via Photoredox Catalysis
Abstract
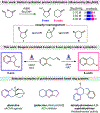
Controlling the regioselectivity of radical cyclizations to favor the 6-endo mode over its kinetically preferred 5-exo counterpart is difficult without introducing substrate prefunctionalization. To address this challenge, we have developed a simple method for reagent controlled regioselective radical cyclization of halogenated N-heterocycles onto pendant olefins. Radical generation occurs under mild photoredox conditions with control of the regioselectivity governed by the rate of hydrogen atom transfer (HAT). Utilizing a polarity-matched thiol-based HAT agent promotes the highly selective formation of the 5-exo cyclization product. Conversely, limiting the solubility of the HAT reagent Hantzsch ester (HEH) leads to selective formation of the thermodynamically favored 6-endo product. This occurs through an initial 5-exo cyclization, with the resulting alkyl radical intermediate undergoing neophyl rearrangement to form the 6-endo product. Development of this switchable catalysis strategy allows for two modes of divergent reactivity to form either the 6-endo or 5-exo product, generating fused N-heteroaromatic/saturated ring systems.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures

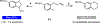
Similar articles
-
Does the Neophyl-like Rearrangement Play a Decisive Role in Intramolecular Cyclization of Iminyl Radicals? A Combined Quantum Chemistry and Numerical Simulation Investigation of the Cyclization Mechanism and Product Distributions of Bicyclic 2-Allyl-2-methyl-2,3-dihydro-1 H-inden-1-iminyl Radical and Several Iminyl Model Compounds.J Org Chem. 2019 Mar 1;84(5):2721-2731. doi: 10.1021/acs.joc.8b03134. Epub 2019 Feb 11. J Org Chem. 2019. PMID: 30695635
-
5-Endo-trig radical cyclizations: disfavored or favored processes?J Am Chem Soc. 2002 Sep 11;124(36):10765-72. doi: 10.1021/ja0261731. J Am Chem Soc. 2002. PMID: 12207532
-
Controlling the regiochemistry of radical cyclizations.Chem Rec. 2006;6(1):23-31. doi: 10.1002/tcr.20069. Chem Rec. 2006. PMID: 16470801 Review.
-
Kinetic study of the 7-endo selective radical cyclization of N-tert-butyl-o-bromobenzylmethacryl amides: kinetic investigation of the cyclization and 1,7-hydrogen transfer of aromatic radicals.J Org Chem. 2013 Apr 19;78(8):3961-71. doi: 10.1021/jo400326b. Epub 2013 Mar 27. J Org Chem. 2013. PMID: 23489331
-
4-Exo-Trig Radical Cyclization: A Promising Approach to Four-Membered Rings.Chemistry. 2025 Apr 9;31(21):e202500206. doi: 10.1002/chem.202500206. Epub 2025 Mar 11. Chemistry. 2025. PMID: 39996614 Review.
Cited by
-
Visible-Light-Driven Carboxylative 1,2-Difunctionalization of C=C Bonds with Tetrabutylammonium Oxalate.ACS Cent Sci. 2024 Nov 15;11(1):46-56. doi: 10.1021/acscentsci.4c01464. eCollection 2025 Jan 22. ACS Cent Sci. 2024. PMID: 39866692 Free PMC article.
-
Unified short syntheses of oxygenated tricyclic aromatic diterpenes by radical cyclization with a photoredox catalyst.Commun Chem. 2023 Aug 21;6(1):169. doi: 10.1038/s42004-023-00979-2. Commun Chem. 2023. PMID: 37604953 Free PMC article.
-
Radical Group Transfer of Vinyl and Alkynyl Silanes Driven by Photoredox Catalysis.J Org Chem. 2023 Sep 1;88(17):12451-12463. doi: 10.1021/acs.joc.3c01213. Epub 2023 Aug 15. J Org Chem. 2023. PMID: 37581630 Free PMC article.
-
Discovery and Development of an Aerobic Radical Hydroxyarylation Reaction Using Aryl Halides, Olefins, and O2.Org Lett. 2025 May 2;27(17):4491-4495. doi: 10.1021/acs.orglett.5c00968. Epub 2025 Apr 17. Org Lett. 2025. PMID: 40245444 Free PMC article.
References
-
- Beckwith ALJ, Regio- and stereo-selectivity in radical reactions. Tetrahedron. 1981, 37, 3073–3100.
- Beckwith ALJ; Schiesser CH, Regio- and stereo-selectivity of alkenyl radical ring closure: A theoretical study. Tetrahedron. 1985, 41, 3925–3941.
-
- Beckwith ALJ; Blair IA; Phillipou G, Subsituent effects on the cyclization of hex-5-enyl radical. Tetrahedron Lett. 1974, 15, 2251–2254.
- Beckwith ALJ; Lawrence T, The effect of non-bonded interactions on the regioselectivity of cyclization of the hex-5-enyl radical. J. Chem Soc., Perkin Trans. 1979, 1535–1539.
-
- Ishibashi H, Controlling the regiochemistry of radical cyclizations. Chem. Rec 2006, 6, 23–31. - PubMed
- Gómez AM,; Company MD; Uriel C; Valverde S; López JC, 6-endo versus 5-exo radical cyclization: streamlined syntheses of carbohexopyranoses and derivatives by 6-endo-trig radical cyclization. Tetrahedron Lett. 2007, 48, 1645–1649.
-
- Beckwith ALJ; O’Shea DM, Kinetics and mechanism of some vinyl radical cyclisations. Tetrahedron Lett. 1986, 27, 4525–4528.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

