Decarbonylative Pd-Catalyzed Suzuki Cross-Coupling for the Synthesis of Structurally Diverse Heterobiaryls
- PMID: 35200025
- PMCID: PMC9069322
- DOI: 10.1021/acs.orglett.2c00267
Decarbonylative Pd-Catalyzed Suzuki Cross-Coupling for the Synthesis of Structurally Diverse Heterobiaryls
Abstract
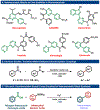
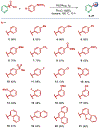
Heteroaromatic biaryls are core scaffolds found in a plethora of pharmaceuticals; however, their direct synthesis by the Suzuki cross-coupling is limited to heteroaromatic halide starting materials. Here, we report a direct synthesis of diverse nitrogen-containing heteroaromatic biaryls by Pd-catalyzed decarbonylative Suzuki cross-coupling of widely available heterocyclic carboxylic acids with arylboronic acids. The practical and modular nature of this cross-coupling enabled the straightforward preparation of >45 heterobiaryl products using pyridines, pyrimidines, pyrazines, and quinolines in excellent yields. We anticipate that the modular nature of this protocol will find broad application in medicinal chemistry and drug discovery research.
Figures



Similar articles
-
Decarbonylative Suzuki-Miyaura Cross-Coupling of Aroyl Chlorides.Org Lett. 2020 Aug 21;22(16):6434-6440. doi: 10.1021/acs.orglett.0c02250. Epub 2020 Aug 10. Org Lett. 2020. PMID: 32806154 Free PMC article.
-
Direct one-pot synthesis of primary 4-amino-2,3-diaryl-quinolines via Suzuki-Miyaura cross-coupling of 2-aryl-4-azido-3-iodoquinolines with arylboronic acids.Molecules. 2011 Oct 25;16(11):8958-72. doi: 10.3390/molecules16118958. Molecules. 2011. PMID: 22027952 Free PMC article.
-
Catalytic and highly regioselective cross-coupling of aromatic C-H substrates.J Am Chem Soc. 2007 Oct 3;129(39):11904-5. doi: 10.1021/ja074395z. Epub 2007 Sep 11. J Am Chem Soc. 2007. PMID: 17845047 No abstract available.
-
Suzuki-miyaura cross-coupling in acylation reactions, scope and recent developments.Molecules. 2013 Jan 17;18(1):1188-213. doi: 10.3390/molecules18011188. Molecules. 2013. PMID: 23344208 Free PMC article. Review.
-
The Liebeskind-Srogl cross-coupling reaction towards the synthesis of biologically active compounds.Eur J Med Chem. 2025 Jun 5;290:117526. doi: 10.1016/j.ejmech.2025.117526. Epub 2025 Mar 20. Eur J Med Chem. 2025. PMID: 40184777 Review.
References
-
- Baumann M; Baxendale IR An overview of the synthetic routes to the best-selling drugs containing 6-membered heterocycles. Beilstein J. Org. Chem 2013, 9, 2265–2319. - PMC - PubMed
- World Health Organization. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances 2018. https://apps.who.int/iris/handle/10665/275695 (accessed, Jan 24, 2022). - PMC - PubMed
- Arora A; Scholar EM Role of Tyrosine Kinase Inhibitors in Cancer Therapy. J. Pharmacol. Exp. Ther 2005, 315, 971–979. - PubMed
- Morphy R Selectively Nonselective Kinase Inhibition: Striking the Right Balance. J. Med. Chem 2010, 53, 1413–1437. - PubMed
- Berg S; Bergh M; Hellberg S; Hogdin K; Lo-Alfredsson Y; Soderman P; von Berg S; Weigelt T; Ormo M; Xue Y; Tucker J; Neelissen J; Jerning E; Nilsson Y; Bhat R Discovery of novel potent and highly selective glycogen synthase kinase-3beta (GSK3beta) inhibitors for Alzheimer’s disease: design, synthesis, and characterization of pyrazines. J. Med. Chem 2012, 55, 9107–9119. - PubMed
- Tan C; Gilligan D; Pacey S Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer. Lancet Oncol. 2015, 16, e447–e459. - PubMed
-
- Miyaura N; Yanagi T; Suzuki A The Palladium-Catalyzed Cross-Coupling Reaction of Phenylboronic Acid with Haloarenes in the Presence of Bases. Synth. Commun 1981, 11, 513–519.
- Miyaura N; Suzuki A Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev 1995, 95, 2457–2483
-
-
For a recent comprehensive review, see:
- Lu H; Yu T-Y; Xu P-F; Wei H Selective Decarbonylation via Transition-Metal-Catalyzed Carbon–Carbon Bond Cleavage. Chem. Rev 2021, 121, 365–411. - PubMed
-
For earlier studies, see:
- Dzik WI; Lange PP; Gooßen LJ Carboxylates as sources of carbon nucleophiles and electrophiles: comparison of decarboxylative and decarbonylative pathways. Chem. Sci 2012, 3, 2671–2678.
- Guo L; Rueping M Decarbonylative Cross-Couplings: Nickel Catalyzed Functional Group Interconversion Strategies for the Construction of Complex Organic Molecules. Acc. Chem. Res 2018, 51, 1185–1195. - PubMed
- Liu C; Szostak M Decarbonylative Cross-Coupling of Amides. Org. Biomol. Chem 2018, 16, 7998–8010. - PubMed
- Zhao Q; Szostak M Redox-neutral decarbonylative cross-couplings coming of age. ChemSusChem 2019, 12, 2983–2987. - PubMed
- Wang Z; Wang X; Nishihara Y Nickel or Palladium-Catalyzed Decarbonylative Transformations of Carboxylic Acid Derivative. Chem. Asian J 2020, 15, 1234–1247. - PubMed
-
-
- Becht J-M; Catala C; Le Drian C; Wagner A Synthesis of Biaryls via Decarboxylative Pd-Catalyzed Cross-Coupling Reaction. Org. Lett 2007, 9, 1781–1783. - PubMed
- Yanagisawa S; Ueda K; Taniguchi T; Itami K Potassium t-Butoxide Alone Can Promote the Biaryl Coupling of Electron-Deficient Nitrogen Heterocycles and Haloarenes. Org. Lett 2008, 10, 4673–4676. - PubMed
- Nandi D; Jhou Y-M; Lee J-Y; Kuo B-C; Liu C-Y; Huang P-W; Lee HM Pd(0)-Catalyzed Decarboxylative Coupling and Tandem C-H Arylation/Decarboxylation for the Synthesis of Heteroaromatic Biaryls. J. Org. Chem 2012, 77, 9384–9390. - PubMed
- Cornella J; Larrosa I Decarboxylative carbon-carbon bond-forming transformations of (hetero)aromatic carboxylic acids. Synthesis 2012, 44, 653–656.
- Wei Y; Hu P; Zhang M; Su W Metal-Catalyzed Decarboxylative C–H Functionalization. Chem. Rev 2017, 117, 8864–8907. - PubMed
-
Even in gold catalysis decarbonylative reactions are known:
- Bucher J; Stößer T; Rudolph M; Rominger F; Hashmi ASK CO Extrusion in Homogeneous Gold Catalysis: Reactivity of Gold Acyl Species Generated through Water Addition to Gold Vinylidenes. Angew. Chem. Int. Ed 2015, 54, 1666–1670. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

