Strategies for Improving Small-Molecule Biosensors in Bacteria
- PMID: 35200325
- PMCID: PMC8869690
- DOI: 10.3390/bios12020064
Strategies for Improving Small-Molecule Biosensors in Bacteria
Abstract
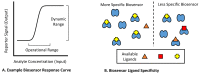
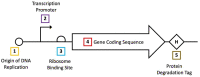
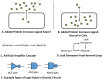
In recent years, small-molecule biosensors have become increasingly important in synthetic biology and biochemistry, with numerous new applications continuing to be developed throughout the field. For many biosensors, however, their utility is hindered by poor functionality. Here, we review the known types of mechanisms of biosensors within bacterial cells, and the types of approaches for optimizing different biosensor functional parameters. Discussed approaches for improving biosensor functionality include methods of directly engineering biosensor genes, considerations for choosing genetic reporters, approaches for tuning gene expression, and strategies for incorporating additional genetic modules.
Keywords: bacterial biosensor; biosensor engineering; genetic circuits; genetic engineering; protein engineering; whole-cell biosensor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
In vivo biosensors: mechanisms, development, and applications.J Ind Microbiol Biotechnol. 2018 Jul;45(7):491-516. doi: 10.1007/s10295-018-2004-x. Epub 2018 Jan 29. J Ind Microbiol Biotechnol. 2018. PMID: 29380152
-
Synthetic biology for microbial heavy metal biosensors.Anal Bioanal Chem. 2018 Feb;410(4):1191-1203. doi: 10.1007/s00216-017-0751-6. Epub 2017 Nov 28. Anal Bioanal Chem. 2018. PMID: 29184994 Review.
-
Recent advances in the analytical strategies of microbial biosensor for detection of pollutants.Chemosphere. 2022 Nov;306:135515. doi: 10.1016/j.chemosphere.2022.135515. Epub 2022 Jun 27. Chemosphere. 2022. PMID: 35772520 Review.
-
Modular tuning engineering and versatile applications of genetically encoded biosensors.Crit Rev Biotechnol. 2022 Nov;42(7):1010-1027. doi: 10.1080/07388551.2021.1982858. Epub 2021 Oct 6. Crit Rev Biotechnol. 2022. PMID: 34615431 Review.
-
Bacterial signaling systems as platforms for rational design of new generations of biosensors.Curr Opin Biotechnol. 2012 Oct;23(5):766-72. doi: 10.1016/j.copbio.2012.05.003. Epub 2012 Jun 2. Curr Opin Biotechnol. 2012. PMID: 22658939 Review.
Cited by
-
Achieving spatially precise diagnosis and therapy in the mammalian gut using synthetic microbial gene circuits.Front Bioeng Biotechnol. 2022 Sep 2;10:959441. doi: 10.3389/fbioe.2022.959441. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36118573 Free PMC article. Review.
-
Innovative applications and research advances of bacterial biosensors in medicine.Front Microbiol. 2025 Apr 23;16:1507491. doi: 10.3389/fmicb.2025.1507491. eCollection 2025. Front Microbiol. 2025. PMID: 40336836 Free PMC article. Review.
-
Improvement of a highly sensitive and specific whole-cell biosensor by adding a positive feedback amplifier.Synth Syst Biotechnol. 2023 Mar 23;8(2):292-299. doi: 10.1016/j.synbio.2023.03.007. eCollection 2023 Jun. Synth Syst Biotechnol. 2023. PMID: 37090062 Free PMC article.
-
Development and Characterization of Indole-Responsive Whole-Cell Biosensor Based on the Inducible Gene Expression System from Pseudomonas putida KT2440.Int J Mol Sci. 2022 Apr 22;23(9):4649. doi: 10.3390/ijms23094649. Int J Mol Sci. 2022. PMID: 35563040 Free PMC article.
-
Continuous Directed Evolution of a Feedback-Resistant Arabidopsis Arogenate Dehydratase in Plantized Escherichia coli.ACS Synth Biol. 2023 Jan 20;12(1):43-50. doi: 10.1021/acssynbio.2c00511. Epub 2022 Dec 19. ACS Synth Biol. 2023. PMID: 36534785 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

