Lysine-PEGylated Cytochrome C with Enhanced Shelf-Life Stability
- PMID: 35200354
- PMCID: PMC8869816
- DOI: 10.3390/bios12020094
Lysine-PEGylated Cytochrome C with Enhanced Shelf-Life Stability
Abstract
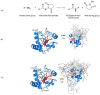
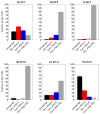
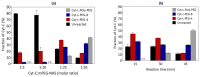
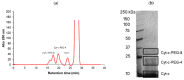
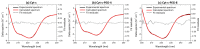
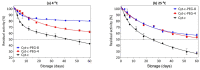
Cytochrome c (Cyt-c), a small mitochondrial electron transport heme protein, has been employed in bioelectrochemical and therapeutic applications. However, its potential as both a biosensor and anticancer drug is significantly impaired due to poor long-term and thermal stability. To overcome these drawbacks, we developed a site-specific PEGylation protocol for Cyt-c. The PEG derivative used was a 5 kDa mPEG-NHS, and a site-directed PEGylation at the lysine amino-acids was performed. The effects of the pH of the reaction media, molar ratio (Cyt-c:mPEG-NHS) and reaction time were evaluated. The best conditions were defined as pH 7, 1:25 Cyt-c:mPEG-NHS and 15 min reaction time, resulting in PEGylation yield of 45% for Cyt-c-PEG-4 and 34% for Cyt-c-PEG-8 (PEGylated cytochrome c with 4 and 8 PEG molecules, respectively). Circular dichroism spectra demonstrated that PEGylation did not cause significant changes to the secondary and tertiary structures of the Cyt-c. The long-term stability of native and PEGylated Cyt-c forms was also investigated in terms of peroxidative activity. The results demonstrated that both Cyt-c-PEG-4 and Cyt-c-PEG-8 were more stable, presenting higher half-life than unPEGylated protein. In particular, Cyt-c-PEG-8 presented great potential for biomedical applications, since it retained 30-40% more residual activity than Cyt-c over 60-days of storage, at both studied temperatures of 4 °C and 25 °C.
Keywords: bioconjugation; cytochrome-c; long-term stability; lysine PEGylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Vazquez-Duhalt R. Cytochrome c as a biocatalyst. J. Mol. Catal. B Enzym. 1999;7:241–249. doi: 10.1016/S1381-1177(99)00033-8. - DOI
-
- Santiago-Rodríguez L., Méndez J., Flores-Fernandez G.M., Pagán M., Rodríguez-Martínez J.A., Cabrera C.R., Griebenow K. Enhanced stability of a nanostructured cytochrome c biosensor by PEGylation. J. Electroanal. Chem. 2011;663:1–7. doi: 10.1016/j.jelechem.2011.09.011. - DOI
-
- Zhang B., Luo Z., Liu J., Ding X., Li J., Cai K. Cytochrome c end-capped mesoporous silica nanoparticles as redox-responsive drug delivery vehicles for liver tumor-targeted triplex therapy in vitro and in vivo. J. Control. Release. 2014;192:192–201. doi: 10.1016/j.jconrel.2014.06.037. - DOI - PubMed
-
- Morales-Cruz M., Figueroa C.M., González-Robles T., Delgado Y., Molina A., Méndez J., Morales M., Griebenow K. Activation of caspase-dependent apoptosis by intracellular delivery of cytochrome c-based nanoparticles. J. Nanobiotechnology. 2014;12:33. doi: 10.1186/s12951-014-0033-9. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- #2016/22065-5/São Paulo Research Foundation
- #2018/25994-2/São Paulo Research Foundation
- CAPES/Brazil, Project 001/Brazilian Coordination for the Improvement of Higher Education Personnel
- CNPq/Brazil, Fellowship #301832/2017-0/National Council for Scientific and Technological Development
- FCT/Portugal, POCI-01-0145-FEDER-007679/Portuguese Foundation for Science and Technology
LinkOut - more resources
Full Text Sources

