An Immersible Microgripper for Pancreatic Islet and Organoid Research
- PMID: 35200420
- PMCID: PMC8869445
- DOI: 10.3390/bioengineering9020067
An Immersible Microgripper for Pancreatic Islet and Organoid Research
Abstract
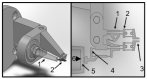
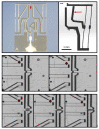
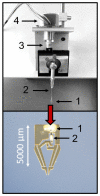
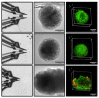
To improve the predictive value of in vitro experimentation, the use of 3D cell culture models, or organoids, is becoming increasingly popular. However, the current equipment of life science laboratories has been developed to deal with cell monolayers or cell suspensions. To handle 3D cell aggregates and organoids in a well-controlled manner, without causing structural damage or disturbing the function of interest, new instrumentation is needed. In particular, the precise and stable positioning in a cell bath with flow rates sufficient to characterize the kinetic responses to physiological or pharmacological stimuli can be a demanding task. Here, we present data that demonstrate that microgrippers are well suited to this task. The current version is able to work in aqueous solutions and was shown to position isolated pancreatic islets and 3D aggregates of insulin-secreting MIN6-cells. A stable hold required a gripping force of less than 30 μN and did not affect the cellular integrity. It was maintained even with high flow rates of the bath perfusion, and it was precise enough to permit the simultaneous microfluorimetric measurements and membrane potential measurements of the single cells within the islet through the use of patch-clamp electrodes.
Keywords: SU-8; cytosolic calcium; electrophysiology; microgripper; organoids; pancreatic islet.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Langer G. Implementation and Use of State-of-the-Art. In: Nielsch U., Fuhrmann U., Jaroch S., editors. New Approaches to Drug Discovery. Vol. 232. Springer; Cham/Basel, Switzerland: 2015. pp. 171–190. - DOI
-
- Garcés-Schröder M., Leester-Schädel M., Schulz M., Böl M., Dietzel A. Micro-Gripper: A new concept for a monolithic single-cell manipulation device. Sensors Actuators A Phys. 2015;236:130–139. doi: 10.1016/j.sna.2015.10.024. - DOI
LinkOut - more resources
Full Text Sources

