Drug Delivery Challenges and Current Progress in Nanocarrier-Based Ocular Therapeutic System
- PMID: 35200463
- PMCID: PMC8871777
- DOI: 10.3390/gels8020082
Drug Delivery Challenges and Current Progress in Nanocarrier-Based Ocular Therapeutic System
Abstract
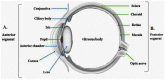
Drug instillation via a topical route is preferred since it is desirable and convenient due to the noninvasive and easy drug access to different segments of the eye for the treatment of ocular ailments. The low dose, rapid onset of action, low or no toxicity to the local tissues, and constrained systemic outreach are more prevalent in this route. The majority of ophthalmic preparations in the market are available as conventional eye drops, which rendered <5% of a drug instilled in the eye. The poor drug availability in ocular tissue may be attributed to the physiological barriers associated with the cornea, conjunctiva, lachrymal drainage, tear turnover, blood-retinal barrier, enzymatic drug degradation, and reflex action, thus impeding deeper drug penetration in the ocular cavity, including the posterior segment. The static barriers in the eye are composed of the sclera, cornea, retina, and blood-retinal barrier, whereas the dynamic barriers, referred to as the conjunctival and choroidal blood flow, tear dilution, and lymphatic clearance, critically impact the bioavailability of drugs. To circumvent such barriers, the rational design of the ocular therapeutic system indeed required enriching the drug holding time and the deeper permeation of the drug, which overall improve the bioavailability of the drug in the ocular tissue. This review provides a brief insight into the structural components of the eye as well as the therapeutic challenges and current developments in the arena of the ocular therapeutic system, based on novel drug delivery systems such as nanomicelles, nanoparticles (NPs), nanosuspensions, liposomes, in situ gel, dendrimers, contact lenses, implants, and microneedles. These nanotechnology platforms generously evolved to overwhelm the troubles associated with the physiological barriers in the ocular route. The controlled-drug-formulation-based strategic approach has considerable potential to enrich drug concentration in a specific area of the eye.
Keywords: drug delivery; hydrogel; implant; microneedle; nanomicelles; nanoparticles; ocular therapeutic system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

