Catalytic Reduction of Environmental Pollutants with Biopolymer Hydrogel Cross-Linked Gelatin Conjugated Tin-Doped Gadolinium Oxide Nanocomposites
- PMID: 35200466
- PMCID: PMC8871642
- DOI: 10.3390/gels8020086
Catalytic Reduction of Environmental Pollutants with Biopolymer Hydrogel Cross-Linked Gelatin Conjugated Tin-Doped Gadolinium Oxide Nanocomposites
Abstract
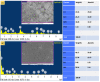
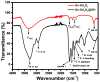
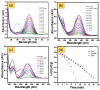
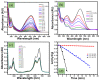
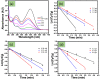
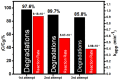
In the present study, a biopolymer nanocomposite hydrogel based on gelatin and tin-doped gadolinium oxide (Sn-Gd2O3@GH) was prepared for the efficient reduction of water pollutants. The method of Sn-Gd2O3@GH preparation consisted of two steps. A Sn-Gd2O3 nanomaterial was synthesized by a hydrothermal method and mixed with a hot aqueous solution (T > 60 °C) of gelatin polymer, followed by cross-linking. Due to the presence of abundant functional groups on the skeleton of gelatin, such as carboxylic acid (-COOH) and hydroxyl (-OH), it was easily cross-linked with formaldehyde. The structure, morphology, and composition of Sn-Gd2O3@GH were further characterized by the FESEM, XRD, EDX, and FTIR techniques. The FESEM images located the distribution of the Sn-Gd2O3 nanomaterial in a GH matrix of 30.06 nm. The XRD patterns confirmed the cubic crystalline structure of Gd2O3 in a nanocomposite hydrogel, while EDS elucidated the elemental composition of pure Sn-Gd2O3 powder and cross-linked the Sn-Gd2O3@GH samples. The synthesized Sn-Gd2O3@GH nanocomposite was used for the removal of different azo dyes and nitrophenols (NPs). It exhibited an efficient catalytic reduction of Congo red (CR) with a reaction rate of 9.15 × 10-1 min-1 with a strong NaBH4-reducing agent. Moreover, the Sn-Gd2O3@GH could be easily recovered by discharging the reduced (colourless) dye, and it could be reused for a fresh cycle.
Keywords: Sn-Gd2O3@GH; azo dye; catalytic reduction; gelatin hydrogel; nanocomposite; nitrophenols.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- Devi L.G., Kumar S.G., Reddy K.M., Munikrishnappa C. Photo degradation of Methyl Orange an azo dye by Advanced Fenton Process using zero valent metallic iron: Influence of various reaction parameters and its degradation mechanism. J. Hazard. Mater. 2009;164:459–467. doi: 10.1016/j.jhazmat.2008.08.017. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

