Antibacterial Hydrogels Derived from Poly(γ-glutamic acid) Nanofibers
- PMID: 35200501
- PMCID: PMC8871545
- DOI: 10.3390/gels8020120
Antibacterial Hydrogels Derived from Poly(γ-glutamic acid) Nanofibers
Abstract
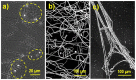
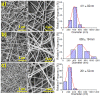
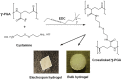
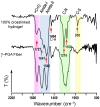
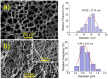
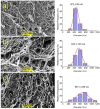
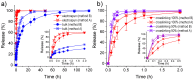
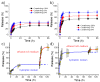
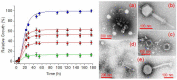
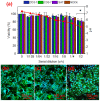
Biocompatible hydrogels with antibacterial properties derived from γ-polyglutamic acid (γ-PGA) were prepared from bulk and electrospun nanofibers. The antibacterial drugs loaded in these hydrogels were triclosan (TCS), chlorhexidine (CHX) and polyhexamethylene biguanide (PHMB); furthermore, bacteriophages were loaded as an alternative antibacterial agent. Continuous and regular γ-PGA nanofibers were successfully obtained by the electrospinning of trifluoroacetic acid solutions in a narrow polymer concentration range and restricted parameter values of flow rate, voltage and needle-collector distance. Hydrogels were successfully obtained by using cystamine as a crosslinking agent following previous published procedures. A closed pore structure was characteristic of bulk hydrogels, whereas an open but structurally consistent structure was found in the electrospun hydrogels. In this case, the morphology of the electrospun nanofibers was drastically modified after the crosslinking reaction, increasing their diameter and surface roughness according to the amount of the added crosslinker. The release of TCS, CHX, PHMB and bacteriophages was evaluated for the different samples, being results dependent on the hydrophobicity of the selected medium and the percentage of the added cystamine. A high efficiency of hydrogels to load bacteriophages and preserve their bactericide activity was demonstrated too.
Keywords: antibacterial properties; bacteriophages; drug release; electrospinning; hydrogels; nanofibers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Fabrication and morphology control of electrospun poly(γ-glutamic acid) nanofibers for biomedical applications.Colloids Surf B Biointerfaces. 2012 Jan 1;89:254-64. doi: 10.1016/j.colsurfb.2011.09.029. Epub 2011 Sep 21. Colloids Surf B Biointerfaces. 2012. PMID: 21982215
-
Polybiguanide (PHMB) loaded in PLA scaffolds displaying high hydrophobic, biocompatibility and antibacterial properties.Mater Sci Eng C Mater Biol Appl. 2015 May;50:74-84. doi: 10.1016/j.msec.2015.01.100. Epub 2015 Feb 2. Mater Sci Eng C Mater Biol Appl. 2015. PMID: 25746248
-
Surface-Modified Carboxylated Cellulose Nanofiber Hydrogels for Prolonged Release of Polyhexamethylene Biguanide Hydrochloride (PHMB) for Antimicrobial Applications.Polymers (Basel). 2023 Aug 28;15(17):3572. doi: 10.3390/polym15173572. Polymers (Basel). 2023. PMID: 37688198 Free PMC article.
-
Recent trends in electrospinning of polymer nanofibers and their applications in ultra thin layer chromatography.Adv Colloid Interface Sci. 2016 Mar;229:1-24. doi: 10.1016/j.cis.2015.12.003. Epub 2015 Dec 17. Adv Colloid Interface Sci. 2016. PMID: 26792019 Review.
-
Biodegradable polymers for electrospinning: towards biomedical applications.Mater Sci Eng C Mater Biol Appl. 2014 Dec;45:659-70. doi: 10.1016/j.msec.2014.04.051. Epub 2014 Apr 28. Mater Sci Eng C Mater Biol Appl. 2014. PMID: 25491875 Review.
Cited by
-
Anti-Pseudomonas Aeruginosa Bacteriophage Loaded Electrospun Fibers for Antibacterial Wound Dressings.Macromol Rapid Commun. 2025 Jul;46(13):e2400744. doi: 10.1002/marc.202400744. Epub 2025 Jan 13. Macromol Rapid Commun. 2025. PMID: 39803850 Free PMC article.
-
A Self-Forming Hydrogel from a Bactericidal Copolymer: Synthesis, Characterization, Biological Evaluations and Perspective Applications.Int J Mol Sci. 2022 Dec 1;23(23):15092. doi: 10.3390/ijms232315092. Int J Mol Sci. 2022. PMID: 36499417 Free PMC article.
-
Poly (γ) glutamic acid: a unique microbial biopolymer with diverse commercial applicability.Front Microbiol. 2024 Feb 13;15:1348411. doi: 10.3389/fmicb.2024.1348411. eCollection 2024. Front Microbiol. 2024. PMID: 38414762 Free PMC article. Review.
References
-
- Langer R. Biomaterials and biomedical engineering. Chem. Eng. Sci. 1995;50:4109–4121. doi: 10.1016/0009-2509(95)00226-X. - DOI
-
- Vedadghavami A., Minooei F., Mohammadi M.H., Khetani S., Rezaei Kolahchi A., Mashayekhan S., Sanati-Nezhad A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017;62:42–63. doi: 10.1016/j.actbio.2017.07.028. - DOI - PubMed
-
- Patel A., Mequanint K. Hydrogel biomaterials. In: Fazel-Rezai R., editor. Hydrogel Biomaterials. InTech Open; London, UK: 2011. pp. 276–295. (Biomedical Engineering-Frontiers and Challenges). Chapter 14. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

