Tacrolimus-Loaded Solid Lipid Nanoparticle Gel: Formulation Development and In Vitro Assessment for Topical Applications
- PMID: 35200510
- PMCID: PMC8871527
- DOI: 10.3390/gels8020129
Tacrolimus-Loaded Solid Lipid Nanoparticle Gel: Formulation Development and In Vitro Assessment for Topical Applications
Abstract
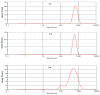
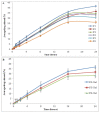
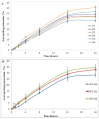
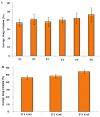
The currently available topical formulations of tacrolimus have minimal and variable absorption, elevated mean disposition half-life, and skin irritation effects resulting in patient noncompliance. In our study, we fabricated tacrolimus-loaded solid lipid nanoparticles (SLNs) that were converted into a gel for improved topical applications. The SLNs were prepared using a solvent evaporation method and characterized for their physicochemical properties. The particle size of the SLNs was in the range of 439 nm to 669 nm with a PDI of ≤0.4, indicating a monodispersed system. The Zeta potential of uncoated SLNs (F1-F5) ranged from -25.80 to -15.40 mV. Those values reverted to positive values for chitosan-decorated formulation (F6). The drug content and entrapment efficiency ranged between 0.86 ± 0.03 and 0.91 ± 0.03 mg/mL and 68.95 ± 0.03 and 83.68 ± 0.04%, respectively. The pH values of 5.45 to 5.53 depict their compatibility for skin application. The surface tension of the SLNs decreased with increasing surfactant concentration that could increase the adherence of the SLNs to the skin. The release of drug from gel formulations was significantly retarded in comparison to their corresponding SLN counterparts (p ≤ 0.05). Both SLNs and their corresponding gel achieved the same level of drug permeation, but the retention of the drug was significantly improved with the conversion of SLNs into their corresponding gel formulation (p ≤ 0.05) due to its higher bioadhesive properties.
Keywords: chitosan; gel; solid lipid nanoparticles; tacrolimus; topical drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Physicochemical Characterization of Chitosan-Decorated Finasteride Solid Lipid Nanoparticles for Skin Drug Delivery.Biomed Res Int. 2022 Aug 6;2022:7792180. doi: 10.1155/2022/7792180. eCollection 2022. Biomed Res Int. 2022. PMID: 35971450 Free PMC article.
-
Astragaloside IV-loaded nanoparticle-enriched hydrogel induces wound healing and anti-scar activity through topical delivery.Int J Pharm. 2013 Apr 15;447(1-2):171-81. doi: 10.1016/j.ijpharm.2013.02.054. Epub 2013 Mar 14. Int J Pharm. 2013. PMID: 23500766
-
Formulation optimization and in vitro skin penetration of spironolactone loaded solid lipid nanoparticles.Colloids Surf B Biointerfaces. 2015 Apr 1;128:473-479. doi: 10.1016/j.colsurfb.2015.02.046. Epub 2015 Mar 5. Colloids Surf B Biointerfaces. 2015. PMID: 25797482
-
Optimization of methazolamide-loaded solid lipid nanoparticles for ophthalmic delivery using Box-Behnken design.J Liposome Res. 2014 Sep;24(3):171-81. doi: 10.3109/08982104.2014.891231. Epub 2014 Mar 10. J Liposome Res. 2014. PMID: 24611687 Review.
-
Solid Lipid Nanoparticles for Topical Drug Delivery: Mechanisms, Dosage Form Perspectives, and Translational Status.Curr Pharm Des. 2020;26(27):3203-3217. doi: 10.2174/1381612826666200526145706. Curr Pharm Des. 2020. PMID: 32452322 Review.
Cited by
-
Recent Advances in Phytochemical-Based Topical Applications for the Management of Eczema: A Review.Int J Mol Sci. 2024 May 15;25(10):5375. doi: 10.3390/ijms25105375. Int J Mol Sci. 2024. PMID: 38791412 Free PMC article. Review.
-
Erythropoietin-PLGA-PEG as a local treatment to promote functional recovery and neurovascular regeneration after peripheral nerve injury.J Nanobiotechnology. 2022 Oct 28;20(1):461. doi: 10.1186/s12951-022-01666-5. J Nanobiotechnology. 2022. PMID: 36307805 Free PMC article.
-
A Reversibly Thermoresponsive, Theranostic Nanoemulgel for Tacrolimus Delivery to Activated Macrophages: Formulation and In Vitro Validation.Pharmaceutics. 2023 Sep 22;15(10):2372. doi: 10.3390/pharmaceutics15102372. Pharmaceutics. 2023. PMID: 37896130 Free PMC article.
-
Formulation and characterization of cholesterol-based nanoparticles of gabapentin protecting from retinal injury.Front Chem. 2024 Oct 21;12:1449380. doi: 10.3389/fchem.2024.1449380. eCollection 2024. Front Chem. 2024. PMID: 39502139 Free PMC article.
-
Tacrolimus: Physicochemical stability challenges, analytical methods, and new formulations.Int J Pharm X. 2024 Sep 15;8:100285. doi: 10.1016/j.ijpx.2024.100285. eCollection 2024 Dec. Int J Pharm X. 2024. PMID: 39328187 Free PMC article. Review.
References
-
- Chu D. Overview of biology, development, and structure of skin. In: Wolff K., Goldsmith L.A., Katz S.I., Gilchrest B.A., Paller A.S., DJ Leffell D.J., editors. Fitzpatrick’s Dermatology in General Medicine. 8th ed. McGraw Hill Medical; New York, NY, USA: 2008.
-
- Del Prado-Audelo M.L., Caballero-Florán I.H., Sharifi-Rad J., Mendoza-Muñoz N., González-Torres M., Urbán-Morlán Z., Florán B., Cortes H., Leyva-Gómez G. Chitosan-decorated nanoparticles for drug delivery. J. Drug Deliv. Sci. Technol. 2020;59:101896. doi: 10.1016/j.jddst.2020.101896. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

