Efficacy of Capecitabine and Temozolomide in Small Bowel (Midgut) Neuroendocrine Tumors
- PMID: 35200546
- PMCID: PMC8870244
- DOI: 10.3390/curroncol29020046
Efficacy of Capecitabine and Temozolomide in Small Bowel (Midgut) Neuroendocrine Tumors
Abstract
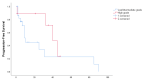
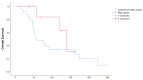
The capecitabine/temozolomide regimen has significant activity in pancreatic NETs; however, data are limited in NETs of the small bowel (midgut). A retrospective study of all patients with metastatic midgut NETs seen at Moffitt Cancer Center between January 2008 and June 2019 treated with CAPTEM was conducted. 32 patients with proven or suspected well-differentiated primary small bowel NETs (excluding duodenum) were identified. 6 patients were found to have a radiographic response (19%), 5 of whom had high-grade disease. Only one patient among 23 with low/intermediate-grade disease responded (4%), whereas the response rate for patients with high-grade disease was 56%. Among patients with low/intermediate-grade disease, 44% discontinued due to poor tolerability. The CAPTEM regimen appears to have an activity in patients with high-grade small bowel NETs and is largely inactive in patients with low/intermediate-grade tumors.
Keywords: capecitabine; captem; carcinoid; midgut; neuroendocrine tumors; small bowel; temozolomide.
Conflict of interest statement
J. Strosberg–Ipsen (Speaker bureau) and Novartis (Consultant); B. Morse and T. Al–Toubah declare no conflict of interest.
Figures
References
-
- Yao J.C., Pavel M., Lombard-Bohas C., Van Cutsem E., Voi M., Brandt U., He W., Chen D., Capdevila J., de Vries E.G.E., et al. Everolimus for the Treatment of Advanced Pancreatic Neuroendocrine Tumors: Overall Survival and Circulating Biomarkers from the Randomized, Phase III RADIANT-3 Study. J. Clin. Oncol. 2016;34:3906–3913. doi: 10.1200/JCO.2016.68.0702. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources