Imaging Spectrum of Intrahepatic Mass-Forming Cholangiocarcinoma and Its Mimickers: How to Differentiate Them Using MRI
- PMID: 35200560
- PMCID: PMC8870737
- DOI: 10.3390/curroncol29020061
Imaging Spectrum of Intrahepatic Mass-Forming Cholangiocarcinoma and Its Mimickers: How to Differentiate Them Using MRI
Abstract
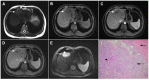
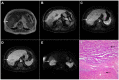
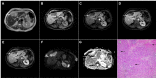
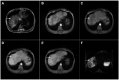
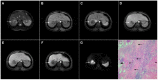
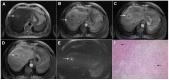
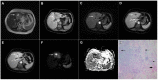
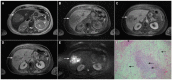
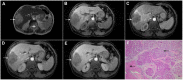
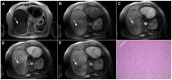
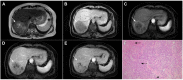
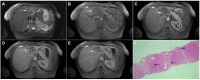
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary hepatic malignancy, with mass-forming growth pattern being the most common. The typical imaging appearance of mass-forming ICC (mICC) consists of irregular ring enhancement in the arterial phase followed by the progressive central enhancement on portal venous and delayed phases. However, atypical imaging presentation in the form of hypervascular mICC might also be seen, which can be attributed to distinct pathological characteristics. Ancillary imaging features such as lobular shape, capsular retraction, segmental biliary dilatation, and vascular encasement favor the diagnosis of mICC. Nevertheless, these radiological findings may also be present in certain benign conditions such as focal confluent fibrosis, sclerosing hemangioma, organizing hepatic abscess, or the pseudosolid form of hydatid disease. In addition, a few malignant lesions including primary liver lymphoma, hemangioendothelioma, solitary hypovascular liver metastases, and atypical forms of hepatocellular carcinoma (HCC), such as scirrhous HCC, infiltrative HCC, and poorly differentiated HCC, may also pose a diagnostic dilemma by simulating mICC in imaging studies. Diffusion-weighted imaging and the use of hepatobiliary contrast agents might be helpful for differential diagnosis in certain cases. The aim of this manuscript is to provide a comprehensive overview of mICC imaging features and to describe useful tips for differential diagnosis with its potential mimickers.
Keywords: magnetic resonance imaging; mass-forming cholangiocarcinoma; mimickers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
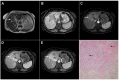
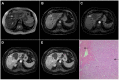
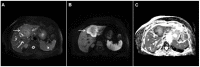
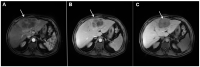






References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

