Diffusion-Weighted MRI in Patients with Testicular Tumors-Intra- and Interobserver Variability
- PMID: 35200570
- PMCID: PMC8871139
- DOI: 10.3390/curroncol29020071
Diffusion-Weighted MRI in Patients with Testicular Tumors-Intra- and Interobserver Variability
Abstract
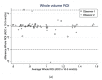
In general, magnetic resonance (MR) diffusion-weighted imaging (DWI) has shown potential in clinical settings. In testicles parenchyma, the DW imaging helps differentiate and characterize benign from malignant lesions. Placement and size of the region of interest (ROI) may affect the ADC value. Therefore, the aim of this study was to investigate the intra- and interobserver variability in testicular tumors when measuring ADC using various types of regions of interest (ROI). Two observers performed the ADC measurements in testicular lesions based on three ROI methods: (1) whole volume, (2) round, and (3) small sample groups. Intra- and interobserver variability was analyzed for all ROI methods using intraclass correlation coefficients (ICC) and bland-altman plots. The two observers performed the measurements twice, three months apart. A total of 26 malignant testicle tumors were included. Interobserver agreement was excellent in tumor length (ICC = 0.98) and tumor width (ICC = 0.98). In addition, intraobserver agreement was excellent in tumor length (ICC = 0.98) and tumor width (ICC = 0.99). The whole volume interobserver agreement in the first reading was excellent (ICC = 0.93). Round ADC had an excellent (ICC = 0.93) and fair (ICC = 0.58) interobserver agreement, in the first and second reading, respectively. Interobserver agreement in ADC small ROIs was good (ICC = 0.87), and good (ICC = 0.78), in the first and second reading, respectively. Intraobserver agreement varied from fair, good to excellent agreement. The ROI method showed varying inter- and intraobserver agreement in ADC measurement. Using multiple small ROI conceded the highest interobserver variability, and, thus, the whole volume or round seem to be the preferable methods.
Keywords: ADC; DWI; interobserver; region of interest; testicular tumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Pedersen M.R., Osther P.J., Nissen H.D., Vedsted P., Møller H., Rafaelsen S.R. Elastography and diffusion-weighted MRI in patients with testicular microlithiasis, normal testicular tissue, and testicular cancer: An observational study. Acta Radiol. 2019;60:535–541. doi: 10.1177/0284185118786063. - DOI - PubMed
-
- Schueller-Weidekamm C., Kaserer K., Schueller G., Scheuba C., Ringl H., Weber M., Czerny C., Herneth A.M. Can Quantitative Diffusion-Weighted MR Imaging Differentiate Benign and Malignant Cold Thyroid Nodules? Initial Results in 25 Patients. Am. J. Neuroradiol. 2009;30:417–422. doi: 10.3174/ajnr.A1338. - DOI - PMC - PubMed
-
- Nerad E., Pizzi A.D., Lambregts D.M.J., Maas M., Wadhwani S., Bakers F.C.H., Bosch H.C.M.V.D., Beets-Tan R.G.H., Lahaye M.J. The Apparent Diffusion Coefficient (ADC) is a useful biomarker in predicting metastatic colon cancer using the ADC-value of the primary tumor. PLoS ONE. 2019;14:e0211830. doi: 10.1371/journal.pone.0211830. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

