Biological Activity and Stability of Aeruginosamides from Cyanobacteria
- PMID: 35200623
- PMCID: PMC8878463
- DOI: 10.3390/md20020093
Biological Activity and Stability of Aeruginosamides from Cyanobacteria
Abstract
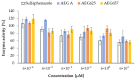
Aeruginosamides (AEGs) are classified as cyanobactins, ribosomally synthesized peptides with post-translational modifications. They have been identified in cyanobacteria of genera Microcystis, Oscillatoria, and Limnoraphis. In this work, the new data on the in vitro activities of three AEG variants, AEG A, AEG625 and AEG657, and their interactions with metabolic enzymes are reported. Two aeruginosamides, AEG625 and AEG657, decreased the viability of human breast cancer cell line T47D, but neither of the peptides was active against human liver cancer cell line Huh7. AEGs also did not change the expression of MIR92b-3p, but for AEG625, the induction of oxidative stress was observed. In the presence of a liver S9 fraction containing microsomal and cytosolic enzymes, AEG625 and AEG657 showed high stability. In the same assays, quick removal of AEG A was recorded. The peptides had mild activity against three cytochrome P450 enzymes, CYP2C9, CYP2D6 and CYP3A4, but only at the highest concentration used in the study (60 µM). The properties of AEGs, i.e., cytotoxic activity and in vitro interactions with important metabolic enzymes, form a good basis for further studies on their pharmacological potential.
Keywords: aeruginosamides; cyanopeptides; cytochrome P450 enzymes; cytotoxicity; metabolic stability; miRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Eighteen New Aeruginosamide Variants Produced by the Baltic Cyanobacterium Limnoraphis CCNP1324.Mar Drugs. 2020 Aug 27;18(9):446. doi: 10.3390/md18090446. Mar Drugs. 2020. PMID: 32867236 Free PMC article.
-
Trichormamides C and D, antiproliferative cyclic lipopeptides from the cultured freshwater cyanobacterium cf. Oscillatoria sp. UIC 10045.Bioorg Med Chem. 2015 Jul 1;23(13):3153-62. doi: 10.1016/j.bmc.2015.04.073. Epub 2015 May 1. Bioorg Med Chem. 2015. PMID: 26001342 Free PMC article.
-
Cytotoxic and peptidase inhibitory activities of selected non-hepatotoxic cyclic peptides from cyanobacteria.Biol Chem. 2008 Oct;389(10):1339-46. doi: 10.1515/BC.2008.153. Biol Chem. 2008. PMID: 18713022
-
Cyanobactins-ribosomal cyclic peptides produced by cyanobacteria.Appl Microbiol Biotechnol. 2010 May;86(5):1213-25. doi: 10.1007/s00253-010-2482-x. Epub 2010 Feb 27. Appl Microbiol Biotechnol. 2010. PMID: 20195859 Free PMC article. Review.
-
Inhibitory Effects of Eight Green Tea Catechins on Cytochrome P450 1A2, 2C9, 2D6, and 3A4 Activities.J Pharm Pharm Sci. 2016 Apr-Jun;19(2):188-97. doi: 10.18433/J3MS5C. J Pharm Pharm Sci. 2016. PMID: 27518169 Review.
Cited by
-
Colombian cyanobacteria with cytotoxic activity in cancer cell lines.Heliyon. 2024 Sep 14;10(19):e37851. doi: 10.1016/j.heliyon.2024.e37851. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39416832 Free PMC article.
-
Structure, Toxicity, Prevalence, and Degradation of Six Understudied Freshwater Cyanopeptides.Toxins (Basel). 2025 May 8;17(5):233. doi: 10.3390/toxins17050233. Toxins (Basel). 2025. PMID: 40423316 Free PMC article. Review.
References
-
- Tiwari A.K., Tiwari B.S. Cyanotherapeutics: An emerging field for future drug discovery. Appl Phyco. 2020;1:1–16. doi: 10.1080/26388081.2020.1744480. - DOI
-
- Khalifa S.A.M., Shedid E.S., Saied E.M., Jassbi A.R., Jamebozorgi F.H., Rateb M.E., Du M., Abdel-Daim M.M., Kai G.-Y., Al-Hammady M.A.M., et al. Cyanobacteria-from the oceans to the potential biotechnological and biomedical applications. Mar. Drugs. 2021;19:241. doi: 10.3390/md19050241. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

