Chitosan and Hyaluronic Acid Nanoparticles as Vehicles of Epoetin Beta for Subconjunctival Ocular Delivery
- PMID: 35200680
- PMCID: PMC8878220
- DOI: 10.3390/md20020151
Chitosan and Hyaluronic Acid Nanoparticles as Vehicles of Epoetin Beta for Subconjunctival Ocular Delivery
Abstract
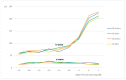
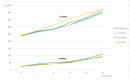
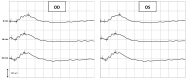
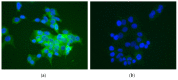
Neuroprotection in glaucoma using epoetin beta (EPOβ) has yielded promising results. Our team has developed chitosan-hyaluronic acid nanoparticles (CS/HA) designed to carry EPOβ into the ocular globe, improving the drug's mucoadhesion and retention time on the ocular surface to increase its bioavailability. In the present in vivo study, we explored the possibility of delivering EPOβ to the eye through subconjunctival administration of chitosan-hyaluronic acid-EPOβ (CS/HA-EPOβ) nanoparticles. Healthy Wistar Hannover rats (n = 21) were split into 7 groups and underwent complete ophthalmological examinations, including electroretinography and microhematocrit evaluations before and after the subconjunctival administrations. CS/HA-EPOβ nanoparticles were administered to the right eye (OD), and the contralateral eye (OS) served as control. At selected timepoints, animals from each group (n = 3) were euthanized, and both eyes were enucleated for histological evaluation (immunofluorescence and HE). No adverse ocular signs, no changes in the microhematocrits (≈45%), and no deviations in the electroretinographies in both photopic and scotopic exams were observed after the administrations (p < 0.05). Intraocular pressure remained in the physiological range during the assays (11-22 mmHg). EPOβ was detected in the retina by immunofluorescence 12 h after the subconjunctival administration and remained detectable until day 21. We concluded that CS/HA nanoparticles could efficiently deliver EPOβ into the retina, and this alternative was considered biologically safe. This nanoformulation could be a promising tool for treating retinopathies, namely optic nerve degeneration associated with glaucoma.
Keywords: chitosan; epoetin beta; erythropoietin; hyaluronic acid; mucoadhesion; nanoparticles; ocular delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











Similar articles
-
Topical ocular delivery of nanoparticles with epoetin beta in Wistar Hannover rats.Sci Rep. 2023 Jan 27;13(1):1559. doi: 10.1038/s41598-023-28845-0. Sci Rep. 2023. PMID: 36707615 Free PMC article.
-
Topical Administration of Mucoadhesive Liposomes-Epoetin-β for Targeting the Ocular Posterior Segment.Mol Pharm. 2025 Apr 7;22(4):2287-2305. doi: 10.1021/acs.molpharmaceut.5c00079. Epub 2025 Mar 18. Mol Pharm. 2025. PMID: 40100128
-
Topical Administration of a Nanoformulation of Chitosan-Hyaluronic Acid-Epoetin Beta in a Rat Model of Glaucoma.Pharmaceuticals (Basel). 2023 Jan 23;16(2):164. doi: 10.3390/ph16020164. Pharmaceuticals (Basel). 2023. PMID: 37259314 Free PMC article.
-
Hyaluronic acid in ocular drug delivery.Carbohydr Polym. 2021 Jul 15;264:118006. doi: 10.1016/j.carbpol.2021.118006. Epub 2021 Mar 29. Carbohydr Polym. 2021. PMID: 33910737 Review.
-
A review on revolutionizing ophthalmic therapy: Unveiling the potential of chitosan, hyaluronic acid, cellulose, cyclodextrin, and poloxamer in eye disease treatments.Int J Biol Macromol. 2024 Jul;273(Pt 2):132700. doi: 10.1016/j.ijbiomac.2024.132700. Epub 2024 Jun 15. Int J Biol Macromol. 2024. PMID: 38879998 Review.
Cited by
-
Stem Cell-Based Therapies for Glaucoma Treatment: A Review Bridging the Gap in Veterinary Patients.Int J Mol Sci. 2024 Dec 30;26(1):232. doi: 10.3390/ijms26010232. Int J Mol Sci. 2024. PMID: 39796087 Free PMC article. Review.
-
Breaking Barriers in Eye Treatment: Polymeric Nano-Based Drug-Delivery System for Anterior Segment Diseases and Glaucoma.Polymers (Basel). 2023 Mar 9;15(6):1373. doi: 10.3390/polym15061373. Polymers (Basel). 2023. PMID: 36987154 Free PMC article. Review.
-
Formulation, Characterisation and Evaluation of the Antihypertensive Peptides, Isoleucine-Proline-Proline and Leucine-Lysine-Proline in Chitosan Nanoparticles Coated with Zein for Oral Drug Delivery.Int J Mol Sci. 2022 Sep 22;23(19):11160. doi: 10.3390/ijms231911160. Int J Mol Sci. 2022. PMID: 36232463 Free PMC article.
-
Novel Biomaterials in Glaucoma Treatment.Biomedicines. 2024 Apr 7;12(4):813. doi: 10.3390/biomedicines12040813. Biomedicines. 2024. PMID: 38672168 Free PMC article. Review.
-
Review: Neuroprotective Nanocarriers in Glaucoma.Pharmaceuticals (Basel). 2024 Sep 10;17(9):1190. doi: 10.3390/ph17091190. Pharmaceuticals (Basel). 2024. PMID: 39338350 Free PMC article. Review.
References
-
- Vidal-Sanz M., Salinas-Navarro M., Nadal-Nicolás F.M., Alarcón-Martínez L., Valiente-Soriano F.J., de Imperial J.M., Avilés-Trigueros M., Agudo-Barriuso M., Villegas-Pérez M.P. Understanding glaucomatous damage: Anatomical and functional data from ocular hypertensive rodent retinas. Prog. Retin. Eye Res. 2012;31:1–27. doi: 10.1016/j.preteyeres.2011.08.001. - DOI - PubMed
-
- Hernández C., Fonollosa A., García-Ramírez M., Higuera M., Catalán R., Miralles A., García-Arumí J., Simó R. Erythropoietin is expressed in the human retina and it is highly elevated in the vitreous fluid of patients with diabetic macular edema. Diabetes Care. 2006;29:2028–2033. doi: 10.2337/dc06-0556. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

