Microglia modulate blood flow, neurovascular coupling, and hypoperfusion via purinergic actions
- PMID: 35201268
- PMCID: PMC8932534
- DOI: 10.1084/jem.20211071
Microglia modulate blood flow, neurovascular coupling, and hypoperfusion via purinergic actions
Abstract
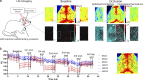
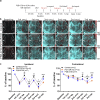
Microglia, the main immunocompetent cells of the brain, regulate neuronal function, but their contribution to cerebral blood flow (CBF) regulation has remained elusive. Here, we identify microglia as important modulators of CBF both under physiological conditions and during hypoperfusion. Microglia establish direct, dynamic purinergic contacts with cells in the neurovascular unit that shape CBF in both mice and humans. Surprisingly, the absence of microglia or blockade of microglial P2Y12 receptor (P2Y12R) substantially impairs neurovascular coupling in mice, which is reiterated by chemogenetically induced microglial dysfunction associated with impaired ATP sensitivity. Hypercapnia induces rapid microglial calcium changes, P2Y12R-mediated formation of perivascular phylopodia, and microglial adenosine production, while depletion of microglia reduces brain pH and impairs hypercapnia-induced vasodilation. Microglial actions modulate vascular cyclic GMP levels but are partially independent of nitric oxide. Finally, microglial dysfunction markedly impairs P2Y12R-mediated cerebrovascular adaptation to common carotid artery occlusion resulting in hypoperfusion. Thus, our data reveal a previously unrecognized role for microglia in CBF regulation, with broad implications for common neurological diseases.
© 2022 Császár et al.
Conflict of interest statement
Disclosures: D. Máthé reported grants from European Union H2020 HCEMM-739593 and from NRDIO, Hungary, TKP-BIOImaging-2020-4.1.1-TKP2020 during the conduct of the study. D. Máthé is CEO and stakeholder of CROmed Ltd. Z. Lenkei reported personal fees from Iconeus outside the submitted work. No other disclosures were reported.
Figures





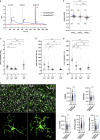
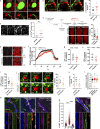
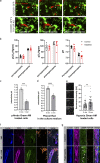
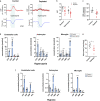

References
-
- Alexander, G.M., Rogan S.C., Abbas A.I., Armbruster B.N., Pei Y., Allen J.A., Nonneman R.J., Hartmann J., Moy S.S., Nicolelis M.A., et al. . 2009. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 63:27–39. 10.1016/j.neuron.2009.06.014. - DOI - PMC - PubMed
-
- Apostolova, I., Wunder A., Dirnagl U., Michel R., Stemmer N., Lukas M., Derlin T., Gregor-Mamoudou B., Goldschmidt J., Brenner W., et al. . 2012. Brain perfusion SPECT in the mouse: Normal pattern according to gender and age. Neuroimage. 63:1807–1817. 10.1016/j.neuroimage.2012.08.038. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

