Sustained defense response via volatile signaling and its epigenetic transcriptional regulation
- PMID: 35201346
- PMCID: PMC9157098
- DOI: 10.1093/plphys/kiac077
Sustained defense response via volatile signaling and its epigenetic transcriptional regulation
Abstract
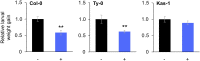
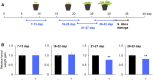
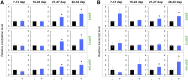
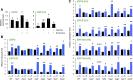
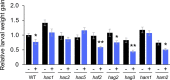
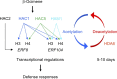
Plants perceive volatiles emitted from herbivore-damaged neighboring plants to urgently adapt or prime their defense responses to prepare for forthcoming herbivores. Mechanistically, these volatiles can induce epigenetic regulation based on histone modifications that alter the transcriptional status of defense genes, but little is known about the underlying mechanisms. To understand the roles of such epigenetic regulation of plant volatile signaling, we explored the response of Arabidopsis (Arabidopsis thaliana) plants to the volatile β-ocimene. Defense traits of Arabidopsis plants toward larvae of Spodoptera litura were induced in response to β-ocimene, through enriched histone acetylation and elevated transcriptional levels of defense gene regulators, including ethylene response factor genes (ERF8 and ERF104) in leaves. The enhanced defense ability of the plants was maintained for 5 d but not over 10 d after exposure to β-ocimene, and this coincided with elevated expression of those ERFs in their leaves. An array of histone acetyltransferases, including HAC1, HAC5, and HAM1, were responsible for the induction and maintenance of the anti-herbivore property. HDA6, a histone deacetylase, played a role in the reverse histone remodeling. Collectively, our findings illuminate the role of epigenetic regulation in plant volatile signaling.
© American Society of Plant Biologists 2022. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Arimura G (2021) Making sense of the way plants sense herbivores. Trends Plant Sci 26: 288–298 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

