Inflawell® improves neutrophil-to-lymphocyte ratio and shortens hospitalization in patients with moderate COVID-19, in a randomized double-blind placebo-controlled clinical trial
- PMID: 35201518
- PMCID: PMC8867130
- DOI: 10.1007/s10787-022-00928-w
Inflawell® improves neutrophil-to-lymphocyte ratio and shortens hospitalization in patients with moderate COVID-19, in a randomized double-blind placebo-controlled clinical trial
Abstract
Aims: COVID-19 is a significant global threat to public health. Despite the availability of vaccines and anti-viral drugs, there is an urgent need for alternative treatments to help prevent and/or manage COVID-19 symptoms and the underlying dysregulated immune response. We hypothesized that administration of Inflawell® syrup, a Boswellia extract formulation enriched for boswellic acids (BAs), can reduce the excessive or persistent inflammation and thereby prevent disease progression. BAs are medicinally activated triterpenoids found in the resins of Boswellia spp., and possess an immense therapeutic potential due to their anti-inflammatory and immunoregulatory activities. We investigated the effect of Inflawell® syrup, on moderate COVID-19 patients along with the current standard of care treatment.
Methods: A randomized placebo-controlled double-blind clinical trial was conducted, following definitive confirmation of COVID-19. Forty-seven hospitalized patients with moderate COVID-19 were enrolled and received either the Inflawell® syrup or placebo. Clinical symptoms and markers of inflammation were evaluated at baseline and completion of the trial.
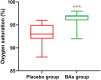
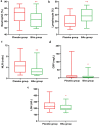
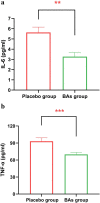
Results: Our clinical trial revealed an increase in the percentage of oxygen saturation level in patients that received the BAs compared to placebo (P < 0.0001). In addition, the average duration of hospitalization was significantly shorter in the BAs group compared with the placebo group (P < 0.04). Concomitantly, some improvement in the clinical symptoms including cough, dyspnea, myalgia, headache, and olfactory and gustatory dysfunction were detected in the BAs group. Hematologic findings showed a significant decrease in the percentage of neutrophils (P < 0.006) and neutrophil-to-lymphocyte ratio (NLR) levels (P < 0.003), associated with a significant increase in the percentage of lymphocytes in the BAs group compared with the placebo (P < 0.002). Additionally, a significant decrease in CRP, LDH, IL - 6 and TNF - α levels was detected in the BAs group. Following the intervention, fewer patients in the BAs group were PCR-positive for COVID-19 compared to placebo, though not statistically significant.
Conclusion: Overall, the treatment with Inflawell® resulted in shorter hospital stay, alleviation of COVID-19 clinical symptoms and decline in the level of pro-inflammatory cytokines.
Trial registration: The trial has been registered in https://www.irct.ir with unique identifier: IRCT20170315033086N10 ( https://en.irct.ir/trial/51631 ). IRCT is a primary registry in the WHO registry network ( https://www.who.int/clinical-trials-registry-platform/network/primary-registries ).
Keywords: Boswellic acids; COVID-19; Clinical trial; Inflammation; Inflawell® syrup; NLR.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

Comment in
-
Comment on "Inflawell, neutrophil-to-lymphocyte ratio and COVID-19".Inflammopharmacology. 2022 Jun;30(3):1129-1130. doi: 10.1007/s10787-022-00970-8. Epub 2022 Mar 22. Inflammopharmacology. 2022. PMID: 35316428 Free PMC article. No abstract available.
Similar articles
-
Glycyrrhizin and boswellic acids, the golden nutraceuticals: multitargeting for treatment of mild-moderate COVID-19 and prevention of post-COVID cognitive impairment.Inflammopharmacology. 2022 Dec;30(6):1977-1992. doi: 10.1007/s10787-022-01062-3. Epub 2022 Sep 22. Inflammopharmacology. 2022. PMID: 36136251 Free PMC article. Review.
-
Boswellic Acids Reduce Systemic Inflammation in Patients with Moderate COVID-19 Through Modulation of NF-κB Pathway.J Diet Suppl. 2025;22(3):365-381. doi: 10.1080/19390211.2025.2468484. Epub 2025 Feb 26. J Diet Suppl. 2025. PMID: 40012185 Clinical Trial.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 11;22(1):42. doi: 10.1186/s13063-020-04998-5. Trials. 2021. PMID: 33430924 Free PMC article.
-
Functional improvement and immune-inflammatory cytokines profile of ischaemic stroke patients after treatment with boswellic acids: a randomized, double-blind, placebo-controlled, pilot trial.Inflammopharmacology. 2019 Dec;27(6):1101-1112. doi: 10.1007/s10787-019-00627-z. Epub 2019 Aug 12. Inflammopharmacology. 2019. PMID: 31407195 Clinical Trial.
Cited by
-
Effects of Boswellia species on viral infections with particular attention to SARS-CoV-2.Inflammopharmacology. 2022 Oct;30(5):1541-1553. doi: 10.1007/s10787-022-01037-4. Epub 2022 Jul 26. Inflammopharmacology. 2022. PMID: 35882701 Free PMC article. Review.
-
Immunomodulatory Role of Plants and Their Constituents on the Management of Metabolic Disorders: An Evidence-Based Review.Drug Des Devel Ther. 2024 Feb 23;18:513-534. doi: 10.2147/DDDT.S442566. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38415194 Free PMC article. Review.
-
Glycyrrhizin and boswellic acids, the golden nutraceuticals: multitargeting for treatment of mild-moderate COVID-19 and prevention of post-COVID cognitive impairment.Inflammopharmacology. 2022 Dec;30(6):1977-1992. doi: 10.1007/s10787-022-01062-3. Epub 2022 Sep 22. Inflammopharmacology. 2022. PMID: 36136251 Free PMC article. Review.
-
The journey of boswellic acids from synthesis to pharmacological activities.Naunyn Schmiedebergs Arch Pharmacol. 2024 Mar;397(3):1477-1504. doi: 10.1007/s00210-023-02725-w. Epub 2023 Sep 23. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 37740772 Free PMC article. Review.
-
Comment on "Inflawell, neutrophil-to-lymphocyte ratio and COVID-19".Inflammopharmacology. 2022 Jun;30(3):1129-1130. doi: 10.1007/s10787-022-00970-8. Epub 2022 Mar 22. Inflammopharmacology. 2022. PMID: 35316428 Free PMC article. No abstract available.
References
-
- Alkhatip AAAMM, Kamel MG, Hamza MK, Farag EM, Yassin HM, Elayashy M, Naguib AA, Wagih M, Abd-Elhay FA-E, Algameel HZ, Yousef MA, Purcell A, Helmy M. The diagnostic and prognostic role of neutrophil-to-lymphocyte ratio in COVID-19: a systematic review and meta-analysis. Expert Rev Mol Diagn. 2021;21(5):505–514. doi: 10.1080/14737159.2021.1915773. - DOI - PMC - PubMed
-
- Baram SM, Karima S, et al. Functional improvement and immune-inflammatory cytokines profile of ischaemic stroke patients after treatment with boswellic acids: a randomized, double-blind, placebo-controlled, pilot trial. Inflammopharmacology. 2019;27(6):1101–1112. doi: 10.1007/s10787-019-00627-z. - DOI - PubMed
-
- Cai J, Li H, Zhang C, Chen Z, Liu H, Lei F, Qin J-J, Liu Y-M, Zhou F, Song X, Zhou J, Zhao Y-C, Wu B, He M, Yang H, Zhu L, Zhang P, Ji Y-X, Zhao G-N, Lu Z, Liu L, Mao W, Liao X, Lu H, Wang D, Xia X, Huang X, Wei X, Xia J, Zhang B-H, Yuan Y, She Z-G, Xu Q, Ma X, Wang Y, Yang J, Zhang X, Zhang X-J, Li H. The neutrophil-to-lymphocyte ratio determines clinical efficacy of corticosteroid therapy in patients with COVID-19. Cell Metab. 2021;33(2):258–269.e253. doi: 10.1016/j.cmet.2021.01.002. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
