Antibiotics in early life and childhood pre-B-ALL. Reasons to analyze a possible new piece in the puzzle
- PMID: 35201533
- PMCID: PMC8777491
- DOI: 10.1007/s12672-022-00465-6
Antibiotics in early life and childhood pre-B-ALL. Reasons to analyze a possible new piece in the puzzle
Abstract
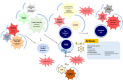
Acute lymphoblastic leukemia (ALL) is the most common pediatric cancer with precursor B-cell ALL (pB-ALL) accounting for ~ 85% of the cases. Childhood pB-ALL development is influenced by genetic susceptibility and host immune responses. The role of the intestinal microbiome in leukemogenesis is gaining increasing attention since Vicente-Dueñas' seminal work demonstrated that the gut microbiome is distinct in mice genetically predisposed to ALL and that the alteration of this microbiome by antibiotics is able to trigger pB-ALL in Pax5 heterozygous mice in the absence of infectious stimuli. In this review we provide an overview on novel insights on the role of the microbiome in normal and preleukemic hematopoiesis, inflammation, the effect of dysbiosis on hematopoietic stem cells and the emerging importance of the innate immune responses in the conversion from preleukemic to leukemic state in childhood ALL. Since antibiotics, which represent one of the most widely used medical interventions, alter the gut microbial composition and can cause a state of dysbiosis, this raises exciting epidemiological questions regarding the implications for antibiotic use in early life, especially in infants with a a preleukemic "first hit". Sheading light through a rigorous study on this piece of the puzzle may have broad implications for clinical practice.
Keywords: Antibiotics; Childhood acute lymphoblastic leukemia; Inflammation; Inherited susceptibility; Innate immune response; Leukemia microenvironment; Microbiome.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Vijayakrishnan J, Qian M, Studd JB, Yang W, Kinnersley B, Law PJ, Broderick P, Raetz EA, Allan J, Pui CH, Vora A, Evans WE, Moorman A, Yeoh A, Yang W, Li C, Bartram CR, Mullighan CG, Zimmerman M, Hunger SP, Schrappe M, Relling MV, Stanulla M, Loh ML, Houlston RS, Yang JJ. Identification of four novel associations for B-cell acute lymphoblastic leukaemia risk. Nat Commun. 2019;10(1):5348. doi: 10.1038/s41467-019-13069-6. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
