Extended-release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults
- PMID: 35201607
- PMCID: PMC8869321
- DOI: 10.1002/14651858.CD012857.pub2
Extended-release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults
Abstract
Background: Attention deficit hyperactivity disorder (ADHD) is a psychiatric diagnosis increasingly used in adults. The recommended first-line pharmacological treatment is central nervous system (CNS) stimulants, such as methylphenidate, but uncertainty remains about its benefits and harms.
Objectives: To assess the beneficial and harmful effects of extended-release formulations of methylphenidate in adults diagnosed with ADHD.
Search methods: We searched CENTRAL, MEDLINE, Embase, nine other databases and four clinical trial registries up to February 2021. We searched 12 drug regulatory databases for clinical trial data up to 13 May 2020. In addition, we cross-referenced all available trial identifiers, handsearched reference lists, searched pharmaceutical company databases, and contacted trial authors.
Selection criteria: Randomised, double-blind, parallel-group trials comparing extended-release methylphenidate formulations at any dose versus placebo and other ADHD medications in adults diagnosed with ADHD.
Data collection and analysis: Two review authors independently extracted data. We assessed dichotomous outcomes as risk ratios (RRs), and rating scales and continuous outcomes as mean differences (MDs) or standardised mean differences (SMDs). We used the Cochrane risk of bias tool to assess risks of bias, and GRADE to assess the certainty of the evidence. We meta-analysed the data using a random-effects model. We assessed three design characteristics that may impair the trial results' 'generalisability'; exclusion of participants with psychiatric comorbidity; responder selection based on previous experience with CNS stimulants; and risk of withdrawal effects. Our prespecified primary outcomes were functional outcomes, self-rated ADHD symptoms, and serious adverse events. Our secondary outcomes included quality of life, ADHD symptoms rated by investigators and by peers such as family members, cardiovascular variables, severe psychiatric adverse events, and other adverse events.
Main results: We included 24 trials (5066 participants), of which 21 reported outcome data for this review. We also identified one ongoing study. We included documents from six drug regulatory agencies covering eight trials. Twenty-one trials had an outpatient setting and three were conducted in prisons. They were primarily conducted in North America and Europe. The median participant age was 36 years. Twelve trials (76% of participants) were industry-sponsored, four (14% of participants) were publicly funded with industry involvement, seven (10% of participants) were publicly funded, and one had unclear funding. The median trial duration was eight weeks. One trial was rated at overall unclear risk of bias and 20 trials were rated at overall high risk of bias, primarily due to unclear blinding of participants and investigators, attrition bias, and selective outcome reporting. All trials were impaired in at least one of the three design characteristics related to 'generalisability'; for example, they excluded participants with psychiatric comorbidity such as depression or anxiety, or included participants only with a previous positive response to methylphenidate, or similar drugs. This may limit the trials' usefulness for clinical practice, as they may overestimate the benefits and underestimate the harms. Extended-release methylphenidate versus placebo (up to 26 weeks) For the primary outcomes, we found very low-certainty evidence that methylphenidate had no effect on 'days missed at work' at 13-week follow-up (mean difference (MD) -0.15 days, 95% confidence interval (CI) -2.11 to 1.81; 1 trial, 409 participants) or serious adverse events (risk ratio (RR) 1.43, CI 95% CI 0.85 to 2.43; 14 trials, 4078 participants), whereas methylphenidate improved self-rated ADHD symptoms (small-to-moderate effect; SMD -0.37, 95% CI -0.43 to -0.30; 16 trials, 3799 participants). For secondary outcomes, we found very low-certainty evidence that methylphenidate improved self-rated quality of life (small effect; SMD -0.15, 95% CI -0.25 to -0.05; 6 trials, 1888 participants), investigator-rated ADHD symptoms (small-to-moderate effect; SMD -0.42, 95% CI -0.49 to -0.36; 18 trials, 4183 participants), ADHD symptoms rated by peers such as family members (small-to-moderate effect; SMD -0.31, 95% CI -0.48 to -0.14; 3 trials, 1005 participants), and increased the risk of experiencing any adverse event (RR 1.27, 95% CI 1.19 to 1.37; 14 trials, 4214 participants). We rated the certainty of the evidence as 'very low' for all outcomes, primarily due to high risk of bias and 'indirectness of the evidence'. One trial (419 participants) had follow-up at 52 weeks and two trials (314 participants) included active comparators, hence long-term and comparative evidence is limited.
Authors' conclusions: We found very low-certainty evidence that extended-release methylphenidate compared to placebo improved ADHD symptoms (small-to-moderate effects) measured on rating scales reported by participants, investigators, and peers such as family members. Methylphenidate had no effect on 'days missed at work' or serious adverse events, the effect on quality of life was small, and it increased the risk of several adverse effects. We rated the certainty of the evidence as 'very low' for all outcomes, due to high risk of bias, short trial durations, and limitations to the generalisability of the results. The benefits and harms of extended-release methylphenidate therefore remain uncertain.
Trial registration: ClinicalTrials.gov NCT00307684 NCT01338818 NCT02675400 NCT04507204 NCT03481959.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Kim Boesen: none known. Asger Sand Paludan‐Müller: none known. Peter C Gøtzsche: none known. Karsten Juhl Jørgensen: none known.
Figures
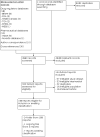
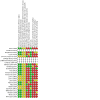





























































Update of
References
References to studies included in this review
Adler 2006 {published and unpublished data}
-
- Adler LA, Zimmerman B, Starr HL, Silber S, Palumbo J, Orman C, et al.Efficacy and safety of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, double-blind, parallel group, dose-escalation study. Journal of Clinical Psychopharmacology 2009;29(3):239-47. [DOI: 10.1097/JCP.0b013e3181a390ce] [PMID: ] - DOI - PubMed
-
- K Boesen.Subject: Cochrane review [personal communication]. Email to: L Adler 18 February 2019.
-
- NCT00326391.A safety and effectiveness study of methylphenidate HCl extended-release tablets in adults with attention deficit hyperactivity disorder [A placebo-controlled, double-blind, parallel-group, dose-titration study to evaluate the efficacy and safety of CONCERTA (methylphenidate HCl) extended-release tablets in adults with attention deficit hyperactivity disorder at doses of 36 mg, 54 mg, 72 mg, 90 mg, or 108 mg per day]. clinicaltrials.gov/ct2/show/NCT00326391 (first received 16 May 2006). [accessed 1 December 2021]
-
- NCT00326391.Clinical Study Report Synopsis (CR011560); 25 July 2007. Available at filehosting.pharmacm.com/DownloadService.ashx?client=CTR_JNJ_6051&st... (accessed 1 December 2021).
-
- Schaeuble B, Orman C, Palumbo JM.P.7.a.003 Efficacy of prolonged-release methylphenidate in a randomized controlled trial in adults with ADHD: secondary endpoints. European Neuropsychopharmacology 2009;19 Suppl 3:S679-80. [DOI: 10.1016/S0924-977X(09)71098-3] - DOI
Asherson 2016 {published and unpublished data}
-
- Asherson P, Johansson L, Holland R, Fahy T, Forester A, Howitt S, et al.Randomised controlled trial of the short-term effects of OROS-methylphenidate on ADHD symptoms and behavioural outcomes in young male prisoners with attention-deficit/hyperactivity disorder (CIAO-II). Trials 2019;20(1):663. [DOI: 10.1186/s13063-019-3705-9] [PMCID: PMC6889577] [PMID: ] - DOI - PMC - PubMed
-
- EUCTR 2015-004271-78.Clinical Study Report (CIAO-II); 26 April 2021 . Available at www.clinicaltrialsregister.eu/ctr-search/rest/download/result/attachment... (accessed 1 December 2021).
-
- EUCTR 2015-004271-78.Randomised controlled trial of the short term effects of OROS-methylphenidate on ADHD symptoms and behavioural outcomes in young male prisoners with attention deficit hyperactivity disorder. Available at clinicaltrialsregister.eu/ctr-search/trial/2015-004271-78/GB (first received 30 March 2016). [accessed 1 December 2021]
-
- ISRCTN16827947.A study of Concerta XL on reducing ADHD symptoms and behavioural problems in adult offenders with ADHD [Randomised controlled trial of the short term effects of OROS-methylphenidate on ADHD symptoms and behavioural outcomes in young male prisoners with attention deficit hyperactivity disorder]. Available at isrctn.com/ISRCTN16827947 (first received 31 May 2016). [accessed 1 December 2021]
-
- K Boesen.Subject: ISRCTN16827947 [personal communication]. Email to: P Asherson 19 February 2019.
Biederman 2003 {published and unpublished data}
-
- Biederman J, Mick E, Fried R, Wilner N, Spencer TJ, Faraone SV.Are stimulants effective in the treatment of executive function deficits? Results from a randomized double blind study of OROS-methylphenidate in adults with ADHD. European Neuropsychopharmacology 2011;21(7):508-15. [DOI: 10.1016/j.euroneuro.2010.11.005] [PMID: ] - DOI - PubMed
-
- Biederman J, Mick E, Spencer T, Surman C, Faraone SV.Is response to OROS-methylphenidate treatment moderated by treatment with antidepressants or psychiatric comorbidity? A secondary analysis from a large randomized double blind study of adults with ADHD. CNS Neuroscience & Therapeutics 2012;18(2):126-32. [DOI: 10.1111/j.1755-5949.2010.00233.x] [PMCID: PMC6493536] [PMID: ] - DOI - PMC - PubMed
-
- Biederman J, Mick E, Surman C, Doyle R, Hammerness P, Harpold T, et al.P.8.059 A randomized, double-blind, placebo-controlled study of OROS methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. European Neuropsychopharmacology 2005;15 Suppl 3:S631-2. [DOI: 10.1016/S0924-977X(05)81339-2] - DOI
Casas 2008 {published and unpublished data}
-
- Bundesinstitut für Arzneimittel und Medizinprodukte.Clinical Study Report Synopsis (42603ATT3013); 18 September 2009. Available at portal.dimdi.de/data/ctr/O-4033974-2-1-897CB7-20131030171751.pdf (accessed 1 December 2021).
-
- Casas M, Rösler M, Kooij JJ, Ginsberg Y, Ramos-Quiroga JA, Heger S, et al.Efficacy and safety of prolonged-release OROS methylphenidate in adults with attention deficit/hyperactivity disorder: a 13-week, randomized, double-blind, placebo-controlled, fixed-dose study. World Journal of Biological Psychiatry 2013;14(4):268-81. [DOI: 10.3109/15622975.2011.600333] [PMID: ] - DOI - PubMed
-
- EUCTR2007-002111-82.A multicenter, randomized, double-blind, placebo-controlled, parallel group, dose-response study to evaluate efficacy and safety of prolonged release (PR) OROS® methylphenidate (54 and 72 mg/day) in adults with attention deficit/hyperactivity disorder. Available at clinicaltrialsregister.eu/ctr-search/trial/2007-002111-82/FR (first received 25 July 2007). [accessed 1 December 2021]
-
- K Boesen.Subject: Cochrane review [personal communication]. Email to: M Casas 17 June 2016.
-
- Kooij JJ, Rösler M, Philipsen A, Wächter S, Dejonckheere J, Van der Kolk A, et al.Predictors and impact of non-adherence in adults with attention-deficit/hyperactivity disorder receiving OROS methylphenidate: results from a randomized, placebo-controlled trial. BMC Psychiatry 2013;13:36. [DOI: 10.1186/1471-244X-13-36] [PMCID: PMC3577504] [PMID: ] - DOI - PMC - PubMed
Chronis‐Tuscano 2004 {published and unpublished data}
-
- Chronis-Tuscano A, Seymour KE, Stein MA, Jones HA, Jiles CD, Rooney ME, et al.Efficacy of osmotic-release oral system (OROS) methylphenidate for mothers with attention-deficit/hyperactivity disorder (ADHD): preliminary report of effects on ADHD symptoms and parenting. Journal of Clinical Psychiatry 2008;69(12):1938-47. [DOI: 10.4088/jcp.v69n1213] [PMID: ] - DOI - PubMed
-
- NCT00318981.Efficacy of Concerta in treating ADHD in mothers of children with ADHD. Available at clinicaltrials.gov/ct2/show/NCT00318981 (first received 27 April 2006). [accessed 1 December 2021]
College Study 2009 {published and unpublished data}
-
- K Boesen.Subject: RE: trial NCT00931398 [personal communication]. Email to: O Bukstein 11 September 2018.
-
- NCT00931398.Treatment of college students with attention-deficit/hyperactivity disorder (ADHD) using OROS methylphenidate [Treatment of college students with ADHD using OROS methylphenidate]. Available at clinicaltrials.gov/ct2/show/NCT00931398 (first received 2 July 2009). [accessed 1 December 2021]
Combine Substudy 2005 {published and unpublished data}
-
- Boesen K.RE: NCT00261872 [personal communication]. Email to: R Anton 12 July 2018.
-
- NCT00261872.Treatment of patients with alcoholism and attention deficit disorder. Available at clinicaltrials.gov/ct2/show/NCT00261872 (first received 5 December 2005) . [accessed 1 December 2021]
Compas 2007 CM {published and unpublished data}
-
- 2006-000222-31.Comparison of structured disorder-specific group psychotherapy plus placebo or methylphenidate versus psychiatric counseling plus placebo or methylphenidate for ADHD in adulthood - a first randomized multicentre study [Vergleich einer strukturierten störungsspezifischen gruppenpsychotherapie plus placebo oder methylphenidat versus einer psychiatrischen beratung plus placebo oder methylphenidat bei ADHS im Erwachsenenalter – eine erste randomisierte MultizenterStudie]. www.clinicaltrialsregister.eu/ctr-search/trial/2006-000222-31/DE (first received 22 September 2006). [accessed 1 December 2021]
-
- Boesen K.Subject: COMPAS trial data [personal communication]. Email to: A Philipsen 13 December 2019.
-
- Endres D, Perlov E, Maier S, Feige B, Nickel K, Goll P, et al.Normal neurochemistry in the prefrontal and cerebellar brain of adults with attention-deficit hyperactivity disorder. Frontiers in Behavioral Neuroscience 2015;9:242. [DOI: 10.3389/fnbeh.2015.00242] [PMCID: PMC4585345] [PMID: ] - DOI - PMC - PubMed
-
- ISRCTN54096201.Effects and mechanisms of psychotherapy in the treatment of attention deficit hyperactivity disorder in adults: the first randomised multicentre study. www.isrctn.com/ISRCTN54096201 (first received 30 April 2007). [accessed 1 December 2021]
Compas 2007 GPT {published and unpublished data}
-
- All references are listed under Compas 2007 CM. N/A.
Ginsberg 2007 {published and unpublished data}
-
- EUCTR 2006002553-80.ADHD in inmates within the Swedish Prison and Probation Service - incidence/diagnosis/treatment/follow-up [ADHD hos intagna inom Kriminalvården förekomst/diagnos/behandling/uppföljning]. Available at www.clinicaltrialsregister.eu/ctr-search/trial/2006-002553-80/SE (first received 15 May 2006). [accessed 1 December 2021]
-
- Ginsberg Y, Hirvikoski T, Grann M, Lindefors N.1465 - Osmotic-release oral system methylphenidate (oros-mph) treatment of adult prison inmates with adhd: a randomised controlled trial with open-label extension. European Psychiatry 2013;28(Suppl 1):1. [DOI: 10.1016/S0924-9338(13)76493-9] - DOI
-
- Ginsberg Y, Hirvikoski T, Grann M, Lindefors N.Long-term functional outcome in adult prison inmates with ADHD receiving OROS-methylphenidate. European Archives of Psychiatry and Clinical Neuroscience 2012;262(8):705-24. [DOI: 10.1007/s00406-012-0317-8] [PMCID: PMC3491195] [PMID: ] - DOI - PMC - PubMed
-
- Ginsberg Y, Långström N, Larsson H, Lindefors N.Long-term treatment outcome in adult male prisoners with attention deficit/hyperactivity disorder: three-year naturalistic follow-up of a 52-week methylphenidate trial. Journal of Clinical Psychopharmacology 2015;35(5):535-43. [DOI: 10.1097/JCP.0000000000000395] [PMID: ] - DOI - PubMed
Goodman 2009 {published and unpublished data}
-
- Goodman DW, Starr HL, Ma YW, Rostain AL, Ascher S, Armstrong RB.Randomized, 6-week, placebo-controlled study of treatment for adult attention-deficit/hyperactivity disorder: individualized dosing of osmotic-release oral system (OROS) methylphenidate with a goal of symptom remission. Journal of Clinical Psychiatry 2017;78(1):105-14. [DOI: 10.4088/JCP.15m10348] [PMID: ] - DOI - PubMed
-
- K Boesen.Subject: Cochrane review [personal communication]. Email to: H Lynn Starr 18 April 2019.
-
- NCT00937040.Adult study/OROS methylphenidate hydrochloride (HCL) (OROS MPH) in adults with attention deficit hyperactivity disorder (ADHD) [A placebo controlled double-blind, parallel group, individualizing dosing study optimizing treatment of adults with attention deficit hyperactivity disorder to an effective response with OROS methylphenidate]. Available at clinicaltrials.gov/ct2/show/NCT00937040 (first received 10 July 2009). [accessed 1 December 2021]
-
- Yale University Open Data Access.Clinical Study Report Synopsis (CONCERTA-ATT-3014); 20 December 2010. Available at yoda.yale.edu/sites/default/files/nct00937040.pdf (accessed 1 December 2021).
-
- Yale University Open Data Access.NCT00937040 [A placebo controlled double-blind, parallel group, individualizing dosing study optimizing treatment of adults with attention deficit hyperactivity disorder to an effective response with OROS methylphenidate]. Available at yoda.yale.edu/nct00937040-placebo-controlled-double-blind-parallel-group... (accessed 1 December 2021).
Huss 2010 {published and unpublished data}
-
- Bundesinstitut für Arzneimittel und Medizinprodukte.Clinical Study Report Synopsis (CRIT124D2302); 7 November 2012. Available at portal.dimdi.de/data/ctr/O-4036816-2-0-E378D3-20190111110032.pdf (accessed 1 December 2021).
-
- Bundesinstitut für Arzneimittel und Medizinprodukte.Clinical Trial Report (CRIT24D2302E1); 5 February 2014. Available at portal.dimdi.de/data/ctr/O-4037228-2-0-A1764D-20140205152929.pdf (accessed 1 December 2021).
-
- Coles T, Coon C, DeMuro C, McLeod L, Gnanasakthy A.Psychometric evaluation of the Sheehan Disability Scale in adult patients with attention-deficit/hyperactivity disorder. Neuropsychiatric Disease and Treatment 2014;10:887-95. [DOI: 10.2147/NDT.S55220] [PMCID: PMC4038420] [PMID: ] - DOI - PMC - PubMed
-
- EUCTR 2010-021533-31.Clinical trial report (CRIT124D2302); 7 November 2012. Available at www.clinicaltrialsregister.eu/ctr-search/rest/download/result/attachment... (accessed 1 December 2021).
-
- EUCTR 2010-021533-31.Efficacy and safety study of methylphenidate hydrochloride modified release in adults with childhood-onset attention deficit/hyperactivity disorder (ADHD) [A 40-week, randomized, double-blind, placebo-controlled, multicenter efficacy and safety study of Ritalin® LA in the treatment of adult patients with childhood-onset ADHD]. Available at clinicaltrialsregister.eu/ctr-search/trial/2010-021533-31/DE (first received 9 November 2011). [accessed 1 December 2021]
Konstenius 2006 {published and unpublished data}
-
- ISRCTN81602628.Clinical trial of long acting methylphenidate in amphetamine addicts with attention deficit hyperactivity disorder (ADHD). Available at www.isrctn.com/ISRCTN81602628 (first received 21 April 2008). [accessed 1 December 2021]
Konstenius 2007 {published and unpublished data}
-
- EUCTR 200600224935.Clinical trial of sustained release methylphenidate for attentiondeficithyperactivitydisorder (ADHD) in adult criminal offenders with amphetamine addiction. Available at www.clinicaltrialsregister.eu/ctr-search/trial/2006-002249-35/SE (first received 9 June 2006). [accessed 1 December 2021]
-
- Franck J, Konstenius M, Jayaram-Lindstrom N, Philips B, Guterstam J.S-36-004. ADHD in drug addiction: a RCT on the feasibility of methylphenidate treatment in criminal amphetamine users. International Journal of Neuropsychopharmacology 2012;15(Suppl 1):41. [DOI: 10.1017/S1461145712000508] - DOI
-
- ISRCTN77940178.Clinical trial of sustained release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adult criminal offenders with amphetamine addiction. Available at www.isrctn.com/ISRCTN77940178 (first received 26 October 2007). [accessed 1 December 2021]
-
- K Boesen.Subject: Cochrane review [personal communication]. Email to: M Konstenius 19 February 2019.
-
- Konstenius M, Jayaram-Lindström N, Guterstam J, Beck O, Philips B, Franck J.Methylphenidate for attention deficit hyperactivity disorder and drug relapse in criminal offenders with substance dependence: a 24-week randomized placebo-controlled trial. Addiction 2014;109(3):440-9. Erratum in: Addition 2014; 109(9): 1571-2. [DOI: 10.1111/add.12369] [PMCID: PMC4226329] [PMID: ] - DOI - PMC - PubMed
Levin 1998a {published and unpublished data}
-
- NCT00061087.Treatment of adult attention deficit hyperactivity disorder (ADHD) in methadone patients - 1 [Treatment of adult ADHD in methadone patients]. Available at clinicaltrials.gov/ct2/show/NCT00061087 (first received 22 May 2003). [accessed 1 December 2021]
-
- NIH RePORTER.5R01DA011444-05. Treatment of adult ADHD in methadone patients. reporter.nih.gov/project-details/6497805 (accessed 1 December 2021).
Levin 1998b {published and unpublished data}
-
- NCT00136734.Methylphenidate treatment for cocaine abuse and ADHD - 1 [Methylphenidate treatment for cocaine abuse and ADHD]. Available at clinicaltrials.gov/ct2/show/NCT00136734 (first received 29 August 2005). [accessed 1 December 2021]
-
- NIH RePORTER.1R01DA011755. Methylphenidate for treatment for cocaine abuse and ADHD. Available at reporter.nih.gov/search/PEPHwbrdG0WBUxKyva6Ozg/project-details/2596880 (accessed 1 December 2021).
Levin 2001 {published and unpublished data}
Medori 2005 {published and unpublished data}
-
- Buitelaar JK, Casas M, Philipsen A, Kooij JJ, Ramos-Quiroga JA, Dejonckheere J, et al.Functional improvement and correlations with symptomatic improvement in adults with attention deficit hyperactivity disorder receiving long-acting methylphenidate. Psychological Medicine 2012;42(1):195-204. [DOI: 10.1017/S0033291711000845] [PMCID: PMC3226157] [PMID: ] - DOI - PMC - PubMed
-
- Buitelaar JK, Kooij JJ, Ramos-Quiroga JA, Dejonckheere J, Casas M, Van Oene JC, et al.Predictors of treatment outcome in adults with ADHD treated with OROS® methylphenidate. Progress in Neuro-Psychopharmacoloy and Biological Psychiatry 2011;35(2):554-60. [DOI: 10.1016/j.pnpbp.2010.12.016] - DOI - PubMed
-
- Buitelaar JK, Sobanski E, Stieglitz RD, Dejonckheere J, Waechter S, Schäuble B.Predictors of placebo response in adults with attention-deficit/hyperactivity disorder: data from 2 randomized trials of osmotic-release oral system methylphenidate. Journal of Clinical Psychiatry 2012;73(8):1097-102. [DOI: 10.4088/JCP.11m07528] [PMID: ] - DOI - PubMed
-
- Buitelaar JK, Trott GE, Hofecker M, Waechter S, Berwaerts J, Dejonkheere J, et al.Long-term efficacy and safety outcomes with OROS-MPH in adults with ADHD. International Journal of Neuropsychopharmacology 2012;15(1):1-13. [DOI: 10.1017/S1461145711001131] [PMCID: PMC3243903] [PMID: ] - DOI - PMC - PubMed
-
- Bundesinstitut für Arzneimittel und Medizinprodukte.Clinical Study Report Synopsis (42603A TT3004); 1 June 2009. Available at portal.dimdi.de/data/ctr/O-2154861-1-1-05F283-20190418181055.pdf (accessed 1 December 2021).
Retz 2008 {published and unpublished data}
-
- Bundesinstitut für Arzneimittel und Medizinprodukte.Clinical Study Report synopsis (2008-000942-29); 22 March 2010 (version 2). Available at portal.dimdi.de/data/ctr/O-2163890-1-0-179913-20170620143824.pdf (accessed August 2021).
-
- EUCTR 2008-00094229.Quality assurance of methylphenidate administration in adults with ADHS [Qualitätssicherung der methylphendidatgabe bei erwachsenen mit ADHS]. Available at www.clinicaltrialsregister.eu/ctr-search/trial/2008-000942-29/DE (first received 17 November 2008). [accessed 1 December 2021]
-
- NCT00730249.Quality assurance of administering methylphenidate in adults with attention deficit hyperactivity disorder (ADHD) - QUMEA [Quality assurance of administering methylphenidate in adults with ADHD]. Available at clinicaltrials.gov/ct2/show/NCT00730249 (first received 8 August 2008). [accessed 1 December 2021]
-
- Retz W, Rösler M, Ose C, Scherag A, Alm B, Philipsen A, et al.Multiscale assessment of treatment efficacy in adults with ADHD: a randomized placebo-controlled, multi-centre study with extended-release methylphenidate. World Journal of Biological Psychiatry 2012;13(1):48-59. [DOI: 10.3109/15622975.2010.540257] [PMCID: PMC3279134] [PMID: ] - DOI - PMC - PubMed
Rösler 2004 {published and unpublished data}
-
- Bundesinstitut für Arzneimittel und Medizinprodukte.Public Assessment Report, Medikinet. portal.dimdi.de/amguifree/am/docoutput/additionalDocDownload.xhtml?dntOb... (accessed prior to 1 August 2021).
-
- Bundesinstitut für Arzneimittel und Medizinprodukte.Public Assessment Report [Öffentlicher bewertungsbericht zur änderungsanzeige vom 14.01.2011. Medikinet adult; 18 July 2011]. Available at portal.dimdi.de/amispb/doc/2011/07/25/2163890/OBFMBD5B836E01CC4AAA.rtf (accessed 1 December 2021).
-
- NCT00619840.Placebo-controlled multi-centre double-blind trial for adults with extended-release methylphenidate for ADHD (EMMA) [Placebo-controlled multi-centre double-blind trial for adults with extended-release methylphenidate for ADHD]. Available at clinicaltrials.gov/ct2/show/NCT00619840 (first received 21 February 2008). [accessed 1 December 2021]
-
- Rösler M, Fischer R, Ammer R, Ose C, Retz W, on behalf of the Study Group.Erratum to: European Archives of Psychiatry and Clinical Neuroscience 2009; 259:120–9. DOI 10.1007/s00406-008-0845-4. European Archives of Psychiatry and Clinical Neuroscience 2009;259:368. [DOI: 10.1007/s00406-009-0005-5] - DOI - PubMed
-
- Rösler M, Fischer R, Ammer R, Ose C, Retz W.A randomised, placebo-controlled, 24-week, study of low-dose extended-release methylphenidate in adults with attention-deficit/hyperactivity disorder. European Archives of Psychiatry and Clinical Neuroscience 2009;259(2):120-9. Erratum in: European Archives of Psychiatry and Clinical Neuroscience 2009;259(6):368. [DOI: 10.1007/s00406-008-0845-4] [PMID: ] - DOI - PubMed
Spencer 2003a {published and unpublished data}
-
- Adler LA, Spencer T, Wang J, Pestreich L, Muniz R.P03.006. Efficacy and safety of once-daily extended-release dexmethylphenidate 30 and 40 mg in adults with ADHD: a double-blind, fixed-dose, placebo-controlled, randomized study. International Journal of Neuropsychopharmacology 2006;9(Suppl 1):S229. [DOI: 10.1017/S1461145706007292] - DOI
-
- Novartis.Clinical Study Report Synopsis (CRIT124E2302); updated 9 September 2005. Available at www.novctrd.com/ctrdweb/trialresult/trialresults/pdf?trialResultId=1623 (accessed 1 December 2021).
-
- US Food and Drug Administration.Drug Approval Package: Focalin XR (dexmethylphenidate hydrochloride) extended release capsules; 2 July 2007. Available at www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021802s000TOC.cfm (accessed 1 December 2021).
Takahashi 2011 {published and unpublished data}
-
- NCT01323192.An efficacy and safety study for JNS001 in adults with attentiondeficit hyperactivity disorder [A double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of JNS001 in adults with attention-deficit/hyperactivity disorder at doses of 18 mg, 36 mg, 54 mg, or 72 mg per day]. Available at clinicaltrials.gov/ct2/show/NCT01323192 (first received 25 March 2011). [accessed 1 December 2021]
-
- Takahashi N, Koh T, Tominaga Y, Saito Y, Kashimoto Y, Matsumura T.A randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of osmotic-controlled release oral delivery system methylphenidate HCl in adults with attention-deficit/hyperactivity disorder in Japan. World Journal of Biological Psychiatry 2014;15(6):488-98. [DOI: 10.3109/15622975.2013.868925] [PMID: ] - DOI - PubMed
-
- Yale University Open Data Access.Clinical Study Report Synopsis (JNS001-JPN-A01); 20 November 2012. Available at yoda.yale.edu/sites/default/files/nct01323192.pdf (accessed 1 December 2021).
-
- Yale University Open Data Access.NCT001323192 [A double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of JNS001 in adults with attention-deficit/hyperactivity disorder at doses of 18 mg, 36 mg, 54 mg, or 72 mg per day]. yoda.yale.edu/nct01323192-double-blind-placebo-controlled-parallel-group... (accessed 1 December 2021).
Weisler 2009 {published and unpublished data}
-
- K Boesen.Subject: Cochrane review [personal communication]. Email to: E Daly 19 February 2019.
-
- NCT00880217.A study to evaluate 3 different doses of JNJ31001074 in the treatment of adults with attentiondeficit/hyperactivity disorder (ADHD) [A randomized, double-blind, placebo- and active-controlled, parallel-group, multicenter study of 3 dosages of JNJ-31001074 in the treatment of adult subjects with attention-deficit/hyperactivity disorder]. Available at clinicaltrials.gov/ct2/show/NCT00880217 (first received 13 April 2009). [accessed 1 December 2021]
-
- Pandina G, Daly E, Gassmann-Mayer C, Cooper K.Exploratory analyses of efficacy by sex and age of histamine3 (h3) receptor antagonist (bavisant) for the treatment of adults with attention deficit hyperactivity disorder. In: 67th Annual Convention of the Society of Biological Psychiatry; 2012 May 3-5; Philadelphia (PA). Philadelphia (PA), 2012. [Poster #203]
-
- Weisler RH, Pandina GJ, Daly EJ, Cooper K, Gassmann-Mayer C, 31001074-ATT2001 Study Investigators.Randomized clinical study of a histamine H3 receptor antagonist for the treatment of adults with attention-deficit hyperactivity disorder. CNS Drugs 2012;26(5):421-34. [DOI: 10.2165/11631990-000000000-00000] [PMID: ] - DOI - PubMed
Weiss 2014 {published and unpublished data}
-
- Health Canada.Product Monograph: Foquest® Submission Control No: 214860; 1 March 2019. Available at pdf.hres.ca/dpd_pm/00049955.PDF (accessed 1 December 2021).
-
- NCT02139124.The efficacy and safety of PRC063 in adult ADHD patients [A phase III, randomized, double-blind, placebo-controlled, parallel-arm, multi-center study measuring the efficacy and safety of PRC-063 in adult ADHD patients]. Available at clinicaltrials.gov/ct2/show/study/NCT02139124 (first received 15 May 2014). [accessed 1 December 2021]
-
- Quinn D, Weiss MD, Donnelly G, Reiz J.Effect of PRC-063 on executive functioning in adults with ADHD treated in a randomized, double-blind, placebo-controlled, multi-center study with a six-month open label extension [poster]. In: 66th Annual Meeting of the Canadian Psychiatric Association; 2016 Sep 22-24; Toronto (ON). 2016.
-
- US Food and Drug Administration.Drug Approval Package: Adhansia XR; 7 February 2019. Available at www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212038Orig1s000TOC.cfm (accessed 1 December 2021).
-
- Weiss MD, Childress AC, Donnelly GA.Efficacy and safety of PRC-063, extended-release multilayer methylphenidate in adults with ADHD including 6-month open-label extension. Journal of Attention Disorders 2021;25(10):1417-28. [DOI: 10.1177/1087054719896853] [PMCID: PMC8273537] [PMID: ] - DOI - PMC - PubMed
Winhusen 2005 {published and unpublished data}
-
- Berlin I, Hu MC, Covey LS, Winhusen T.Attention-deficit/hyperactivity disorder (ADHD) symptoms, craving to smoke, and tobacco withdrawal symptoms in adult smokers with ADHD. Drug and Alcohol Dependence 2012;124(3):268-73. [DOI: 10.1016/j.drugalcdep.2012.01.019] [NIHMSID: NIHMS444432] [PMCID: PMC3605750] [PMID: ] - DOI - PMC - PubMed
-
- Boesen K.Cochrane review [personal communication]. Email to : T Winhusen 18 February 2019.
-
- Covey LS, Hu MC, Green CA, Brigham G, Hurt RD, Adler L, et al.An exploration of site effects in a multisite trial of OROS-methylphenidate for smokers with attention deficit/hyperactivity disorder. American Journal of Drug and Alcohol Abuse 2011;37(5):392-9. [DOI: 10.3109/00952990.2011.596979] [PMCID: PMC3510007] [PMID: ] - DOI - PMC - PubMed
-
- Covey LS, Hu MC, Winhusen T, Lima J, Berlin I, Nunes E.Anxiety and depressed mood decline following smoking abstinence in adult smokers with attention deficit hyperactivity disorder. Journal of Substance Abuse Treatment 2015;59:104-8. [DOI: 10.1016/j.jsat.2015.07.004] [NIHMSID: NIHMS709235] [PMCID: PMC4661105] [PMID: ] - DOI - PMC - PubMed
References to studies excluded from this review
Adler 2011 {published data only}
-
- Adler LA, Orman C, Starr HL, Silber S, Palumbo J, Cooper K, et al.Long-term safety of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder: an open-label, dose-titration, 1-year study. Journal of Clinical Psychopharmacology 2011;31(1):108-14. [DOI: 10.1097/JCP.0b013e318203ea0a] [PMID: ] - DOI - PubMed
Ang 2015 {published data only}
-
- Ang A, Hillhouse M, Jenkins J, Reed S, Ling W.Methylphenidate for methamphetamine use disorders in participants with and without ADHD. Drug and Alcohol Dependence 2015;156:e7. [DOI: 10.1016/j.drugalcdep.2015.07.938] - DOI
Biehl 2016 {published data only}
-
- Biehl SC, Merz CJ, Dresler T, Heupel J, Reichert S, Jacob CP, et al.Increase or decrease of fMRI activity in adult attention deficit/hyperactivity disorder: does it depend on task difficulty? International Journal of Neuropsychopharmacology 2016;19(10):pyw049. [DOI: 10.1093/ijnp/pyw049] [PMCID: PMC5091824] [PMID: ] - DOI - PMC - PubMed
Bink 2014 {published data only}
Bouziane 2019 {published data only}
-
- Bouziane C, Filatova OG, Schrantee A, Caan MW, Vos FM, Reneman L.White matter by diffusion MRI following methylphenidate treatment: a randomized control trial in males with attention-deficit/hyperactivity disorder. Radiology 2019;293(1):186-92. [DOI: 10.1148/radiol.2019182528] [PMID: ] - DOI - PubMed
Buitelaar 2009 {published data only}
-
- Buitelaar JK, Ramos-Quiroga JA, Casas M, Kooij JJ, Niemelä A, Konofal E, et al.Safety and tolerability of flexible dosages of prolonged-release OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Neuropsychiatric Disease and Treatment 2009;5:457-66. [DOI: 10.2147/ndt.s6873] [PMCID: PMC2747385] [PMID: ] - DOI - PMC - PubMed
EUCTR 2005‐004037‐18 {published data only}
-
- EUCTR 2005-004037-18.An open label, multicentre study to evaluate the long term safety of prolonged release (PR) OROS methylphenidate (18, 36, 54, 72 and 90 mg/day) in adults with attention deficit/hyperactivity disorder - Lamda extension. www.clinicaltrialsregister.eu/ctr-search/trial/2005-004037-18/DE (first received 1 December 2005). [accessed September 2021]
EUCTR 2008‐006242‐26 {published data only}
-
- EUCTR 2008-006242-26/DE.Genetic modulation of functional brain activity of attention-deficit/hyperactivity disorder-related working memory processes. www.clinicaltrialsregister.eu/ctr-search/trial/2008-006242-26/DE (first received 7 August 2009).
EUCTR 2010‐023654‐37 {published data only}
-
- EUCTR 2010-023654-37/NL.Ritalin and the developing brain [Effects of methylphenidate on the development of the dopaminergic system in the brain]. www.clinicaltrialsregister.eu/ctr-search/trial/2010-023654-37/NL (first received 1 November 2010).
EUCTR 2011‐000210‐19 {published data only}
-
- EUCTR 2011-000210-19/DE.Six-month open label extension to an efficacy and safety study of Ritalin LA in the treatment of adult patients with childhood-onset ADHD [A 6-month, open-label extension to a 40-week, randomized, double-blind, placebo-controlled, multicenter efficacy and safety study of Ritalin® LA in the treatment of adult patients with childhood-onset ADHD]. www.clinicaltrialsregister.eu/ctr-search/trial/2011-000210-19/DE (first received 7 April 2011).
EUCTR 2012‐000517‐37 {published data only}
-
- EUCTR 2012-000517-37/GB.Clinical trial of Concerta for the treatment of ADHD in adult offenders [A pilot study of Concerta XL in adult offenders with ADHD]. www.clinicaltrialsregister.eu/ctr-search/trial/2012-000517-37/GB (first received 31 October 2012).
EUCTR 2014‐001488‐11 {published data only}
-
- EUCTR 2014-001488-11/SE.Effects of expectations, medication and placebo during the Quantified Behavior Test in patients with untreated ADHD and substance use disorder [Effekten av förväntning, läkemedel och placebo på objektiv och självskattad prestation vid QbTest hos patienter med obehandlad ADHD och substanssyndrom] [Effects of expectations, medication and placebo on objective and self-rated performance during the Quantified Behavior Test in terms of the ADHD core signs hyperactivity, inattention and impulsivity in patients with untreated ADHD and substance use disorder [Effekten av förväntning, aktivt läkemedel och placebo på objektiv och självskattad prestation vid QbTest avseende ADHD-kärnsymtomen hyperaktivitet, ouppmärksamhet och impulsivitet hos patienter med obehandlad ADHD och substanssyndrom]]. www.clinicaltrialsregister.eu/ctr-search/trial/2014-001488-11/SE (first received 16 December 2014).
EUCTR 2017‐000368‐14 {published data only}
-
- EUCTR 2017-000368-14/SE.A clinical trial of methylphenidate and lisdexamphetamine treatment in adults with attention-deficit hyperactivity disorder [En jämförande studie av metylfenidat och lisdexamfetamine hos vuxna personer med ADHD] [A head-to-head randomized clinical trial of methylphenidate and lisdexamphetamine treatment for executive functions and global functioning in adults with ADHD [En jämförande randomiserad klinisk prövning med metylfenidat och lisdexamfetamine behandling med avseende på exekutiva funktioner och global funktionsnivå hos vuxna personer med ADHD]]. www.clinicaltrialsregister.eu/ctr-search/trial/2017-000368-14/SE (first received 30 March 2017).
Geissler 2020 {published data only}
-
- Geissler JM, Vloet TD, Strom N, Jaite C, Graf E, Kappel V, et al.Does helping mothers in multigenerational ADHD also help children in the long run? 2-year follow-up from baseline of the AIMAC randomized controlled multicentre trial. European Child & Adolescent Psychiatry 2020;29(10):1425-39. [DOI: 10.1007/s00787-019-01451-0] [PMID: ] - DOI - PubMed
Häge 2018 {published data only}
-
- Häge A, Alm B, Banaschewski T, Becker K, Colla M, Freitag C, et al.Does the efficacy of parent-child training depend on maternal symptom improvement? Results from a randomized controlled trial on children and mothers both affected by attention-deficit/hyperactivity disorder (ADHD). European Child & Adolescent Psychiatry 2018;27(8):1011-21. [DOI: 10.1007/s00787-018-1109-0] [PMID: ] - DOI - PubMed
Imhof 2009 {published data only}
-
- Imhof L, Nyerod HJ, Filipe HJ, Dejonckheere J, Wachter S, Schauble B, et al.P.1.i.034 Efficacy outcomes during longterm prolonged-release methylphenidate treatment of adults with ADHD. European Neuropsychopharmacology 2009;19(Suppl 3):S353-4. [DOI: 10.1016/S0924-977X(09)70537-1] - DOI
ISRCTN52392534 {published data only}ISRCTN52392534
-
- ISRCTN52392534.The Continuous Performance Test (CPT) study: OROS-methylphenidate efficacy on objective measures [Efficacy of OROS-methylphenidate on specific executive functioning deficits in adults with Attention deficit hyperactivity disorder (ADHD)]. www.isrctn.com/ISRCTN52392534 (first received 18 June 2013).
ISRCTN77828247 {published data only}ISRCTN77828247
-
- ISRCTN77828247.Biphentin effects in attention deficit hyperactivity disorder (ADHD) drivers [Biphentineffects in attention deficit hyperactivity disorder (ADHD) drivers: a randomised, placebo controlled, crossover study of multilayer-release (MLR) methylphenidate on driving performance in adult ADHD patients]. www.isrctn.com/ISRCTN77828247 (first received 13 July 2009).
Jans 2013 {published data only}
-
- Jans T, Graf E, Jacob C, Zwanzger U, Gross-Lesch S, Matthies S, et al.A randomized controlled multicentre trial on the treatment for ADHD in mothers and children: enrolment and basic characteristics of the study sample. ADHD Attention Deficit and Hyperactivity Disorders 2013;5(1):29-40. [DOI: 10.1007/s12402-012-0092-4] [PMID: ] - DOI - PubMed
Levin 2002 {published data only}
Marchant 2010 {published data only}
-
- Marchant BK, Reimherr FW, Halls C, Williams ED, Strong RE.OROS methylphenidate in the treatment of adults with ADHD: a 6-month, open-label, follow-up study. Annals of Clinical Psychiatry 2010;22(3):196-204. [PMID: ] - PubMed
NCT00307684 {published data only}
-
- NCT00307684.Open label, multicentre extension study of protocol 42603ATT3002 to evaluate safety of prolonged release OROS methlyphenidate in adults with attention deficit hyperactivity disorder (ADHD) [An open international multicentre long-term follow up study to evaluate safety of prolonged release OROS methlyphenidate in adults with attention deficit hyperactivity disorder]. www.clinicaltrials.gov/ct2/show/NCT00307684 (first received 28 March 2006). [accessed September 2021]
NCT01338818 {published data only}
-
- NCT01338818.Open-label extension evaluating methylphenidate hydrochloride extended release in adults with attention deficit/hyperactivity disorder [A 6-month, open-label extension to a 40-week, randomized, double-blind, placebo-controlled, multicenter efficacy and safety study of methylphenidate hydrochloride extended release in the treatment of adult patients with childhood-onset ADHD]. www.clinicaltrials.gov/ct2/show/NCT01338818 (first received 20 April 2011).
NCT02675400 {published data only}
-
- NCT02675400.Treatments for fathers with ADHD and their at-risk children (Fathers Too) [Treatments for fathers with attention deficit/hyperactivity disorder (ADHD) and their at-risk children (Fathers Too)]. www.clinicaltrials.gov/ct2/show/NCT02675400 (first received 5 February 2016).
NCT04507204 {published data only}
-
- NCT04507204.Real-world evidence of duration of effect of adhansia XR (extended-release) for treatment of attention-deficit/hyperactivity disorder (ADHD) (RE-DAX) [Real-world evidence of duration of adhansia XR for treatment of ADHD (RE-DAX): an open-label pragmatic study to assess the real-world effectiveness of adhansia XR in treatment of adult and adolescent patients with ADHD in the United States]. www.clinicaltrials.gov/ct2/show/NCT04507204 (first received 11 August 2020).
Olsen 2012 {published data only}
-
- Olsen JL, Reimherr FW, Marchant BK, Wender PH, Robison RJ.The effect of personality disorder symptoms on response to treatment with methylphenidate transdermal system in adults with attention-deficit/hyperactivity disorder. Primary Care Companion for CNS Disorders 2012;14(5):PCC.12m01344. [DOI: 10.4088/PCC.12m01344] [PMCID: PMC3583767] [PMID: ] - DOI - PMC - PubMed
Reimherr 2010 {published data only}
-
- Reimherr FW, Marchant BK, Williams ED, Strong RE, Halls C, Soni P.Personality disorders in ADHD part 3: personality disorder, social adjustment, and their relation to dimensions of adult ADHD. Annals of Clinical Psychiatry 2010;22(2):103-12. [PMID: ] - PubMed
Reimherr 2013 {published data only}
Reimherr 2015 {published data only}
-
- Reimherr FW, Marchant BK, Gift TE, Steans TA, Wender PH.Types of adult attention-deficit hyperactivity disorder (ADHD): baseline characteristics, initial response, and long-term response to treatment with methylphenidate. Attention Deficit and Hyperactivity Disorders 2015;7(2):115-28. [DOI: 10.1007/s12402-015-0176-z] [PMID: ] - DOI - PubMed
Schaeuble 2010 {published data only}
-
- Schaeuble B, Hofecker M, Buitelaar J, Kooij S, Dejonckheere J, Waechter S.P.1.c.025 Longterm safety and efficacy outcomes in adults with ADHD treated with prolonged-release methylphenidate. European Neuropsychopharmacology 2010;20(Suppl 3):S249. [DOI: 10.1016/S0924-977X(10)70312-6] - DOI
Schrantee 2016a {published data only}
-
- Boesen K.Methylphenidate trial [personal communication]. Email to: L Reneman 3 January 2018.
-
- Schrantee A, Tamminga HG, Bouziane C, Bottelier MA, Bron EE, Klein S, et al.474. A randomized clinical trial on the age-dependent effects of methylphenidate on the human dopaminergic system. Biological Psychiatry 2016;79(Suppl 9):S157. [URL: Available at www.sobp.org/journal] - PMC - PubMed
Schrantee 2016b {published data only}
-
- Boesen K.Methylphenidate trial [personal communication]. Email to: L Reneman 3 January 2018.
-
- Schrantee A, Tamminga HG, Bouziane C, Bottelier MA, Bron EE, Mutsaerts H-JM, et al.Age-dependent effects of methylphenidate on the human dopaminergic system in young vs adult patients with attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry 2016;73(9):955-62. [DOI: 10.1001/jamapsychiatry.2016.1572] [PMCID: PMC5267166] [PMID: ] - DOI - PMC - PubMed
Surman 2019 {published data only}
-
- Surman C, Ceranoglu A, Vaudreuil C, Albright B, Uchida M, Yule A, et al.Does l-methylfolate supplement methylphenidate pharmacotherapy in attention-deficit/hyperactivity disorder? Evidence of lack of benefit from a double-blind, placebo-controlled, randomized clinical trial. Journal of Clinical Psychopharmacology 2019;39(1):28-38. [DOI: 10.1097/JCP.0000000000000990] [PMCID: PMC6750952] [PMID: ] - DOI - PMC - PubMed
Walhovd 2020 {published data only}
-
- Walhovd KB, Amlien I, Schrantee A, Rohani DA, Groote I, Bjørnerud A, et al.Methylphenidate effects on cortical thickness in children and adults with attention-deficit/hyperactivity disorder: a randomized clinical trial. AJNR: American Journal of Neuroradiology 2020;41(5):758‐65. [DOI: 10.3174/ajnr.A6560] [PMCID: PMC7228175 (available on 2021-05-01)] [PMID: ] - DOI - PMC - PubMed
Wender 1998 {published data only}
-
- Wender PH.Pharmacotherapy of attention-deficit/hyperactivity disorder in adults. Journal of Clinical Psychiatry 1998;59(Suppl 7):76-9. [PMID: ] - PubMed
Wender 2001 {published data only}
-
- Wender PH, Reimherr FW, Marchant B, Czajkowski L, Sanford ME.A placebo-controlled, long-term trial of methylphenidate in the treatment of adults with ADHD. In: 154th Annual Meeting of the American Psychiatric Association; 2001 May 5-10; New Orleans (LA). 2001.
Williams 2010 {published data only}
-
- Williams ED, Reimherr FW, Marchant BK, Strong RE, Halls C, Soni P, et al.Personality disorder in ADHD Part 1: assessment of personality disorder in adult ADHD using data from a clinical trial of OROS methylphenidate. Annals of Clincal Psychiatry 2010;22(2):84-93. [PMID: ] - PubMed
Zinnow 2018 {published data only}
-
- Zinnow T, Banaschewski T, Fallgatter AJ, Jenkner C, Millenet S, Philipsen-Wiegmann F, et al.ESCAlate - An adaptive treatment approach for adolescents and adults with ADHD [ESCAlate – Ein adaptiver behandlungsansatz für jugendliche und erwachsene mit ADHS]. Zeitschrift für Psychiatrie, Psychologie und Psychotherapie 2018;66(4):219-31. [DOI: 10.1024/1661-4747/a000360] - DOI
References to studies awaiting assessment
Perry 2002 {published data only}
-
- Perry PJ.Bupropion sustained release versus methylphenidate versus placebo in the treatment of adult ADHD. In: 155th Annual Meeting of the American Psychiatric Association; 2002 May 18-23; Philadelphia (PA). 2002. [CENTRAL: CN–00340471]
Spencer 2003b {published data only}
-
- Spencer TJ.Preliminary results of a six-month trial of methylphenidate in adults with ADHD. In: The Promise of Science. The Power of Healing. 156th Annual Meeting of the American Psychiatric Association; 2003 May 17-22; San Francisco (CA). 2003:116. [ABSTRACT #: 54B]
Spencer 2004 {published data only}
-
- Spencer TJ, Kim S, Jiang H.Efficacy of dexmethylphenidate extended release capsules in adults with ADHD. In: Psychotherapy and Psychopharmacology: Dissolving the Mind-Brain Barrier. 157th Annual Meeting of the American Psychiatric Association; 2004 May 1-6; New York (NY). 2004. [ABSTRACT #: NR455]
Wender 2002 {published data only}
-
- Wender PH.A placebo-controlled, long-term trial of methylphenidate in the treatment of adults with ADHD. In: 155th Annual Meeting of the American Psychiatric Association; 2002 May 18-23; Philadelphia (PA). 2002.
Wender 2003a {published data only}
-
- Wender PH, Szajkowski L, Marchant B, Reimherr FW, Sanford E, Eden J.A long-term study of methylphenidate in the treatment of ADHD in adults. In: 156th Annual Meeting of the American Psychiatric Association; 2003 May 17-22; San Francisco (CA). 2003:NR708. [CENTRAL: CN-00592892]
Wender 2003b {published data only}
-
- Wender PH, Reimherr FW, Marchant B, Sanford E, Czajkowski L.A long-term trial of methylphenidate in the treatment of ADHD in adults. In: 156th Annual Meeting of the American Psychiatric Association; 2003 May 17-22 San Francisco (CA). 2003:NR709. [CENTRAL: CN-00592894]
Zhong 2019 {published data only}
-
- Zhong Y, Su Y, Cao Q, Sun L, Wang Y, Yang Z, et al.Machine learning based ADHD medication response prediction. ADHD Attention Deficit and Hyperactivity Disorders 2019;11(Suppl 1):S72. [DOI: 10.1007/s12402-019-00295-7] - DOI
References to ongoing studies
Methacan 2017 {published data only}
-
- EUCTR 2017-000386-77.Effectiveness of extended release methylphenidate in reducing cannabis use in young cannabis-dependent patients with attention deficit hyperactivity disorder (ADHD). Available at www.clinicaltrialsregister.eu/ctr-search/trial/2017-000386-77/FR (first received 22 January 2018). [accessed 1 December 2021]
-
- NCT03481959.Effectiveness of methylphenidate late formula to reduce cannabis use in young cannabis-related patients and attention deficit disorder hyperactivity (METHACAN). Available at clinicaltrials.gov/ct2/show/NCT03481959 (first received 29 March 2018). [accessed 1 December 2021]
Additional references
Agnew‐Blais 2016
-
- Agnew-Blais JC, Polanczyk GV, Danese A, Wertz J, Moffitt TE, Arseneault L.Evaluation of the persistence, remission, and emergence of attention-deficit/hyperactivity disorder in young adulthood. JAMA Psychiatry 2016;73(7):713-20. [DOI: 10.1001/jamapsychiatry.2016.0465] [PMCID: PMC5475268] [PMID: ] - DOI - PMC - PubMed
Anton 2006
APA 1987
-
- American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R). 3rd edition. Washington (DC): American Psychiatric Association, 1987.
APA 2000
-
- American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). 4th edition. Washington (DC): American Psychiatric Association, 2000.
APA 2013
-
- American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th edition. Washington (DC): American Psychiatric Association, 2013.
Asherson 2010
Bailly 2005
-
- Bailly L.Stimulant medication for the treatment of attention-deficit hyperactivity disorder: evidence-b(i)ased practice? Psychiatric Bulletin 2005;29(8):284-7. [DOI: 10.1192/pb.29.8.284] - DOI
Baker 2004
-
- Baker A, Lee NK, Jenner L, editor(s).Models of intervention and care for psychostimulant users, 2nd Edition. Drug Strategy Monograph Series No.: 51 (last updated 2004). health.gov.au/internet/publications/publishing.nsf/Content/drugtreat-pub... (accessed 1 December 2021).
Banaschewski 2016
-
- Banaschewski T, Gerlach M, Becker K, Holtmann M, Döpfner M, Romanos M.Trust, but verify. The errors and misinterpretations in the Cochrane Analysis by OJ Storebo and Colleagues on the efficacy and safety of methylphenidate for the treatment of children and adolescents with ADHD. Zeitschrift fur Kinder - und Jugendpsychiatrie Psychotherapie 2016;44(4):307-14. [DOI: 10.1024/1422-4917/a000433] [PMID: ] - DOI - PubMed
Barkley 2002
Beau‐Lejdstrom 2016
BfArM 2011
-
- Bundesinstitut für Arzneimittel und Medizinprodukte.Öffentlicher Bewertungsbericht [Public Assessment Report] Medikinet adult; 18 July 2011. portal.dimdi.de/amispb/doc/2011/07/25/2163890/OBFMBD5B836E01CC4AAA.rtf (accessed 1 December 2021).
Biederman 2006
Boesen 2017a
-
- Boesen K, Saiz LC, Erviti J, Storebø OJ, Gluud C, Gøtzsche PC, et al.The Cochrane Collaboration withdraws a review on methylphenidate for adults with attention deficit hyperactivity disorder. Evidence-Based Medicine 2017;22(4):143-7. [DOI: 10.1136/ebmed-2017-110716] [PMCID: PMC5537554] [PMID: ] - DOI - PMC - PubMed
Boesen 2021a
-
- Boesen K, Jørgensen KJ, Gøtzsche PC.Clinical trials were missing from regulatory documents of extended-release methylphenidate for ADHD in adults: a case study of public documents. Journal of Clinical Epidemiology 2021 Nov 6 [Epub ahead of print]. [DOI: 10.1016/j.jclinepi.2021.10.027] [PMID: ] - DOI - PubMed
Boesen 2021b
-
- Boesen K, Jørgensen KJ, Gøtzsche PC.The US Food and Drug Administration’s authorisation of Purdue’s controlled-release methylphenidate for adult ADHD: comments on the regulatory practice. Journal of the Royal Society of Medicine 2021;114(8):377-80. [DOI: 10.1177/0141076821994535] [PMCID: PMC8361378 (available on 2024-08-01)] [PMID: ] - DOI - PMC - PubMed
Boesen 2021c
Boesen 2021d
-
- Boesen K, Bruckner T, Dirnagl U, Gøtzsche P, Ioannidis J, Jefferson T, et al.[Open letter] Proposal to the Heads of Medicines Agencies to improve harmonisation of access to Clinical Study Reports across National Competent Authorities; 31 May 2021. Available at www.bihealth.org/fileadmin/QUEST/Dokumente/Boesen_HMA_letter.pdf.
Boesen 2021e
-
- Boesen K.Working towards harmonised access to clinical trial data across European drug regulatory agencies; 31 July 2021. blogs.bmj.com/bmj/2021/07/31/working-towards-harmonised-access-to-clinic... (accessed 1 December 2021).
Bron 2014
-
- Bron TI, Bijlenga D, Boonstra AM, Breuk M, Pardoen WF, Beekman AT, et al.OROS-methylphenidate efficacy on specific executive functioning deficits in adults with ADHD: a randomized, placebo-controlled cross-over study. European Neuropsychopharmacology 2014;24(4):519-28. [DOI: 10.1016/j.euroneuro.2014.01.007] [PMID: ] - DOI - PubMed
Cândido 2021
-
- Cândido RF, De Padua CA, Golder S, Junqueira DR.Immediate-release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD013011. [DOI: 10.1002/14651858.CD013011.pub2] [PMCID: PMC8092481 (available on 2022-01-18)] [PMID: ] - DOI - PMC - PubMed
Castells 2011
Castells 2013
-
- Castells X, Cunill R, Capellà D.Treatment discontinuation with methylphenidate in adults with attention deficit hyperactivity disorder: a meta-analysis of randomized clinical trials. European Journal of Clinical Pharmacolology 2013;69(3):347-56. [DOI: 10.1007/s00228-012-1390-7] [PMID: ] - DOI - PubMed
Castells 2018
Catalá‐López 2017
-
- Catalá-López F, Hutton B, Núñez-Beltrán A, Page MJ, Ridao M, Saint-Gerons DM, et al.The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: a systematic review with network meta-analyses of randomised trials. PLOS One 2017;12(7):e0180355. [DOI: 10.1371/journal.pone.0180355] [PMCID: PMC5507500] [PMID: ] - DOI - PMC - PubMed
Caye 2016
-
- Caye A, Rocha TB, Anselmi L, Murray J, Menezes AM, Barros FC, et al.Attention-deficit/hyperactivity disorder trajectories from childhood to young adulthood: evidence from a birth cohort supporting a late-onset syndrome. JAMA Psychiatry 2016;73(7):705-12. [DOI: 10.1001/jamapsychiatry.2016.0383] [PMID: ] - DOI - PubMed
Challman 2000
-
- Challman TD, Lipsky JJ.Methylphenidate: its pharmacology and uses. Mayo Clinic Proceedings 2000;75(7):711-21. [DOI: 10.4065/75.7.711] [PMID: ] [URL: www.mayoclinicproceedings.org/article/S0025-6196(11)64618-1/pdf] - DOI - PubMed
Chang 2014
Chevance 2020
-
- Chevance A, Ravaud P, Tomlinson A, Berre CL, Teufer B, Touboul S, et al.Identifying outcomes for depression that matter to patients, informal caregivers, and health-care professionals: qualitative content analysis of a large international online survey. Lancet Psychiatry 2020;7(8):692-702. [DOI: 10.1016/S2215-0366(20)30191-7] [PMID: ] - DOI - PubMed
Cho 2007
-
- Cho SC, Hwang JW, Kim BN, Lee HY, Kim HW, Lee JS, et al.The relationship between regional cerebral blood flow and response to methylphenidate in children with attention-deficit hyperactivity disorder: comparison between non-responders to methylphenidate and responders. Journal of Psychiatric Research 2007;41(6):459-65. [DOI: 10.1016/j.jpsychires.2006.05.011] [PMID: ] - DOI - PubMed
Coghill 2004
Coghill 2013
-
- Coghill D, Banaschewski T, Zuddas A, Pelaz A, Gagliano A, Doepfner M.Long-acting methylphenidate formulations in the treatment of attention-deficit/hyperactivity disorder: a systematic review of head-to-head studies. BMC Psychiatry 2013;13:237. [DOI: 10.1186/1471-244X-13-237] [PMCID: PMC3852277] [PMID: ] - DOI - PMC - PubMed
COMET 2021
-
- COMET Initiative: Core Outcome Measures in Effectiveness Trials. www.comet-initiative.org (accessed 1 December 2021).
Conners 1999
-
- Conners CK, Erhardt D, Sparrow EP.Conners’ Adult ADHD Rating Scales (CAARS): Technical Manual. North Tonawanda (NY): Multi-Health Systems Inc, 1999.
Cooper 2011
Cortese 2018
-
- Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, et al.Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry 2018;5(9):727-38. [DOI: 10.1016/S2215-0366(18)30269-4] [PMCID: PMC6109107] [PMID: ] - DOI - PMC - PubMed
Cortese 2018b
-
- Cortese S, Faraone SV, Bernardi S, Wang S, Blanco C.Gender differences in adult attention-deficit/hyperactivity disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Journal of Clinical Psychiatry 2016;77(4):e421-8. [DOI: 10.4088/JCP.14m09630] [PMID: ] - DOI - PubMed
Cortese 2020
Covidence [Computer program]
-
- Covidence .Melbourne, Australia: Veritas Health Innovation, accessed 1 December 2021. Available at www.covidence.org.
Cox 2008
-
- Cox DJ, Moore M, Burket R, Merkel RL, Mikami AY, Kovatchev B.Rebound effects with long-acting amphetamine or methylphenidate stimulant medication preparations among adolescent male drivers with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2008;18(1):1-10. [DOI: 10.1089/cap.2006.0141] [PMID: ] - DOI - PubMed
Cruickshank 2009
Cumyn 2009
Cunill 2016
-
- Cunill R, Castells X, Tobias A, Capellà D.Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology 2016;233(2):187-97. [DOI: 10.1007/s00213-015-4099-3] [PMID: ] - DOI - PubMed
CURIA 2020
-
- Court of Justice of the European Union.PRESS RELEASE No 6/20. Judgments in Case C-175/18 P PTC: Therapeutics International Ltd v European Medicines Agency (EMA), and C-178/18 P MSD Animal Health Innovation and Intervet International v European Medicines Agency (EMA); 22 January 2020. curia.europa.eu/jcms/upload/docs/application/pdf/2020-01/cp200006en.pdf (accessed 1 December 2021).
Dalsgaard 2014
-
- Dalsgaard S, Kvist AP, Leckman JF, Nielsen HS, Simonsen M.Cardiovascular safety of stimulants in children with attention-deficit/hyperactivity disorder: a nationwide prospective cohort study. Journal of Child and Adolescent Psychopharmacology 2014;24(6):302-10. [DOI: 10.1089/cap.2014.0020] [PMCID: PMC4137345] [PMID: ] - DOI - PMC - PubMed
Deeks 2021
-
- Deeks JJ, Higgins JP, Altman DG, editor(s).Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
De Faria 2021
-
- De Faria JC, Duarte LJ, Ferreira LA, Da Silveira VT, De Pádua CM, Perini E.“Real-world” effectiveness of methylphenidate in improving the academic achievement of attention-deficit hyperactivity disorder diagnosed students—a systematic review. Journal of Clinical Pharmacy and Therapeutics 2021 Jul 13 [Epub ahead of print]. [DOI: 10.1111/jcpt.13486] [PMID: ] - DOI - PubMed
De Sonneville 1994
-
- De Sonneville LM, Njiokiktjien C, Bos H.Methylphenidate and information processing. Part 1: differentiation between responders and nonresponders; Part 2: efficacy in responders. Journal of Clinical and Experimental Neuropsychology 1994;16(6):877-97. [DOI: 10.1080/01688639408402700] [PMID: ] - DOI - PubMed
DGPPN 2021
-
- DGPPN, Deutsche Gesellschaft für Kinder- und Jugendpsychiatrie, Psychosomatik und Psychotherapie (DGKJP), Deutsche Gesellschaft für Sozialpädiatrie und Jugendmedizin eV(DGSPJ).Long version of the interdisciplinary evidence- and consensus-based (S3) guideline 'Attention deficit/hyperactivity control (ADHD) in childhood, adolescence and adulthood'. AWMF registration number 028-045 [Langfassung der interdisziplinären evidenz- und konsensbasierten (S3) Leitlinie 'Aufmerksamkeitsdefizit-/Hyperaktivitätsöstrung (ADHS) im Kindes-, Jugend- und Erwachsenenalter'. AWMF-Registernummer 028-045]. www.dgppn.de/_Resources/Persistent/6f514fd31d75221054f1d880bda9637728e2b... (accessed 15 September 2021).
Doernberg 2016
Dyer 2020
Egger 1997
Elder 2010
Elia 1991
Elliott 2020
-
- Elliott J, Johnston A, Husereau D, Kelly SE, Eagles C, Charach A, et al.Pharmacologic treatment of attention deficit hyperactivity disorder in adults: a systematic review and network meta-analysis. PLOS One 2020;15(10):e0240584. [DOI: 10.1371/journal.pone.0240584] [PMCID: PMC7577505] [PMID: ] - DOI - PMC - PubMed
EMA 2018
-
- European Medicines Agency.European Medicines Agency policy on access to documents. Policy/0043; October 2018. Available at www.ema.europa.eu/en/documents/other/policy/0043-european-medicines-agen... (accessed 15 September 2021).
EMA 2019
-
- European Medicines Agency.European Medicines Agency policy on publication of clinical data for medicinal products for human use. Policy/0070; 21 March 2019. Available at www.ema.europa.eu/en/documents/other/european-medicines-agency-policy-pu... (accessed 15 September 2021).
Epstein 2014
Epstein 2016
Evans 2010
Faraone 2004
Faraone 2006
Faraone 2010
Faraone 2020a
-
- Faraone SV, Rostain AL, Montano CB, Mason O, Antshel KM, Newcorn JH.Systematic review: nonmedical use of prescription stimulants: risk factors, outcomes, and risk reduction strategies. Journal of the American Academy of Child & Adolescent Psychiatry 2020;59(1):100-12. [DOI: 10.1016/j.jaac.2019.06.012] [PMID: ] - DOI - PubMed
Faraone 2020b
-
- Faraone SV, Banaschewski T, Coghill D, Cortese S, Newcorn JH, Moscibrodzki P, et al.Application for inclusion to the 23rd expert committee on the selection and use of Essential Medicines for children: methylphenidate hydrochloride; 25 November 2020. Available at cdn.who.int/media/docs/default-source/essential-medicines/2021-eml-exper... (accessed 1 December 2021).
Faraone 2021
-
- Faraone SV, Banaschewski T, Coghill D, Zeng Y, Biederman J, Bellgrove MA, et al.The World Federation of ADHD International Consensus Statement: 208 evidence-based conclusions about the disorder. Neuroscience and Biobehavioral Reviews 2021;128:789-818. [DOI: 10.1016/j.neubiorev.2021.01.022] [PMCID: PMC8328933] [PMID: ] - DOI - PMC - PubMed
Fayyad 2017
-
- Fayyad J, Sampson NA, Hwang I, Adamowski T, Aguilar-Gaxiola S, Al-Hamzawi A, et al.The descriptive epidemiology of DSM-IV Adult ADHD in the World Health Organization World Mental Health Surveys. ADHD Attention Deficit and Hyperactivity Disorders 2017;9(1):47-65. [DOI: 10.1007/s12402-016-0208-3] [NIHMSID: NIHMS850481] [PMCID: PMC5325787] [PMID: ] - DOI - PMC - PubMed
FDA 2007
-
- US Food and Drug Administration Center for Drug Evaluation and Research.Drug Approval Package: Focalin XR; 2 July 2007 . Available at www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021802s000TOC.cfm (accessed 1 December 2021).
FDA 2017
-
- US Food and Drug Administration.Prescribing information: Concerta®; revised January 2017 . Available at www.accessdata.fda.gov/drugsatfda_docs/label/2017/021121s038lbl.pdf (accessed 1 December 2021).
FDA 2019
-
- US Food and Drug Administration.Enrichment strategies for clinical trials to support approval of human drugs and biological products; March 2019 . Available at www.fda.gov/media/121320/download (accessed 1 December 2021).
Foquest 2019
-
- Purdue Pharma.Product Monograph Foquest. Submission control no.: 214860; 1 March 2019 . Available at pdf.hres.ca/dpd_pm/00049955.PDF (accessed 1 December 2021).
Franke 2013
Glenngård 2013
-
- Glenngård AH, Hjemgren J, Thomsen PH, Tvedten T.Patient preferences and willingness-to-pay for ADHD treatment with stimulants using discrete choice experiment (DCE) in Sweden, Denmark and Norway. Nordic Journal of Psychiatry 2013;67(5):351-9. [DOI: 10.3109/08039488.2012.748825] [PMID: ] - DOI - PubMed
GradePro GDT [Computer program]
-
- GRADEpro GDT.Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 1 August 2021. Available at gradepro.org.
Grosz‐Lesch 2013
Gualtieri 1985
Gøtzsche 2011
-
- Gøtzsche PC, Jørgensen AW.Opening up data at the European Medicines Agency. BMJ 2011;342:d2686. [DOI: 10.1136/bmj.d2686 ] [PMID: ] - PubMed
Habel 2011
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA, editor(s).Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
-
- Higgins JP, Eldridge S, Li T, editor(s).Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Holick 2009
Huss 2017
ICH 2003
-
- ICH Expert Working Group, International conference on harmonisation of technical requirements for registration of pharmaceutical human use.ICH Harmonised Tripartite Guideline. Post-approval safety data management: definitions and standards for expedited reporting E2D; 12 November 2003. Available at database.ich.org/sites/default/files/E2D_Guideline.pdf (accessed 1 December 2021).
Iversen 2006
Jacob 2007
-
- Jacob CP, Romanos J, Dempfle A, Heine M, Windemuth-Kieselbach C, Kruse A, et al.Co-morbidity of adult attention-deficit/hyperactivity disorder with focus on personality traits and related disorders in a tertiary referral center. European Archives of Psychiatry and Clinical Neuroscience 2007;257(6):309-17. [DOI: 10.1007/s00406-007-0722-6] [PMID: ] - DOI - PubMed
Jensen 2007
Jørgensen 2020
-
- Jørgensen L, Gøtzsche PC, Jefferson T.Benefits and harms of the human papillomavirus (HPV) vaccines: comparison of trial data from clinical study reports with corresponding trial register entries and journal publications. Systematic Reviews 2020;9(1):42. [DOI: 10.1186/s13643-020-01300-1] - DOI - PMC - PubMed
Kazda 2021
-
- Kazda L, Bell K, Thomas R, McGeechan K, Sims R, Barratt A.Overdiagnosis of attention-deficit/hyperactivity disorder in children and adolescents: a systematic scoping review. JAMA Network Open 2021;4(4):e215335. [DOI: 10.1001/jamanetworkopen.2021.5335] [PMCID: PMC8042533] [PMID: ] - DOI - PMC - PubMed
Kessler 2006
-
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, et al.The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. American Journal of Psychiatry 2006;163(4):716-23. [DOI: 10.1176/ajp.2006.163.4.716] [PMCID: PMC2859678] [PMID: ] - DOI - PMC - PubMed
Kirkham 2018
Koesters 2009
Kooij 2010
Kooij 2019
Kortekaas‐Rijlaarsdam 2019
Krinzinger 2019
-
- Krinziger H, Hall CL, Groom MJ, Ansari MT, Banaschewski T, Buitelaar JK, et al.Neurological and psychiatric adverse effects of long-term methylphenidate treatment in ADHD: a map of the current evidence. Neuroscience & Biobehavioral Reviews 2019;107:945-68. [DOI: 10.1016/j.neubiorev.2019.09.023] [PMID: ] - DOI - PubMed
Kuperman 2001
Lachin 2016
Lago 1994
Lange 2010
Layton 2018
Lerner 2019
Leufkens 2021
Liang 2018
-
- Liang EF, Lim SZ, Tam WW, Ho CS, Zhang MW, McIntyre RS, et al.The effect of methylphenidate and atomoxetine on heart rate and systolic blood pressure in young people and adults with attention-deficit hyperactivity disorder (ADHD): systematic review, meta-analysis, and meta-regression. International Journal of Environmental Research and Public Health 2018;15(8):1789. [DOI: 10.3390/ijerph15081789] [PMCID: PMC6121294] [PMID: ] - DOI - PMC - PubMed
Lichtenstein 2012
Ling 2014
Linssen 2014
Lohr 2021
Maldonado 2013
Marcovitch 2004
Maund 2014
Mayo‐Wilson 2017
Mayo‐Wilson 2017a
-
- Mayo-Wilson E, Li T, Fusco N, Bertizzolo L, Canner JK, Cowley T, et al.Cherry-picking by trialists and meta-analysts can drive conclusions about intervention efficacy. Journal of Clinical Epidemiology 2017;91:95-110. - PubMed
Mayo‐Wilson 2018
McDonagh 2011
-
- McDonagh MS, Peterson K, Thakurta S, Low A.Drug Class Review: Pharmacologic Treatments for Attention Deficit Hyperactivity Disorder, Final Update 4 Report. Portland (OR): Oregon Health & Science University, 2011. [BOOKSHELF ID: NBK84419] [PMID: ] - PubMed
McDonagh 2015
-
- McDonagh M, Peterson K, Holzhammer B.Drug Class Review: Pharmacologic Treatments for Attention Deficit Hyperactivity Disorder, Final Update 5 Report. Portland (OR): Oregon Health & Science University, 2015. - PubMed
MEB 2016
-
- Medicines Evaluation Board.Public Assessment Report. Scientific discussion. Ritalin LA 10 mg, 20 mg, 30 mg, 40 mg and 60 mg, modified-release capsules (methylphenidate hydrochloride). NL License RVG: 116377, 116379-116385; 20 October 2016. Available at db.cbg-meb.nl/Pars/h116381.pdf.
MedDRA
-
- Medical Dictionary for Regulatory Activities.MedDRA. www.meddra.org (accessed 4 November 2020).
Meszaros 2009
MHRA 2010
-
- Medicines Healthcare products Regulatory Agency.Public Assessment Report. Mutual Recognition Procedure. Concerta® XL 27 mg prolonged-release tablets (methylphenidate hydrochloride), UK/H/0544/004/MR. UK licence no: PL 00242/0400; July 2010 (updated November 2013). mhraproductsproduction.blob.core.windows.net/docs/e16cca8676c72aff9c51ed... (accessed 1 December 2021).
Mick 2008
Mick 2013
Moffitt 2015
-
- Moffitt TE, Houts R, Asherson P, Belsky DW, Corcoran DL, Hammerle M, et al.Is Adult ADHD a childhood-onset neurodevelopmental disorder? Evidence from a four-decade longitudinal cohort study. American Journal of Psychiatry 2015;172(10):967-77. [DOI: 10.1176/appi.ajp.2015.14101266] [PMCID: PMC4591104] [PMID: ] - DOI - PMC - PubMed
Moher 2009
Molina 2007
-
- Molina BS, Flory K, Hinshaw SP, Greiner AR, Arnold LE, Swanson JM, et al.Delinquent behavior and emerging substance use in the MTA at 36 months: prevalence, course, and treatment effects. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(8):1028-40. [DOI: 10.1097/chi.0b013e3180686d96] [PMID: ] - DOI - PubMed
Molina 2009
-
- Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, et al.The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. Journal of the American Academy of Child and Adolescent Psychiatry 2009;48(5):484-500. [CLINICALTRIALS.GOV: NCT00000388] [DOI: 10.1097/CHI.0b013e31819c23d0] [PMCID: PMC3063150 ] [PMID: ] - DOI - PMC - PubMed
Moncrieff 2010
Moncrieff 2011
-
- Moncrieff J, Timimi S.Critical analysis of the concept of adult attention-deficit hyperactivity disorder. Psychiatrist 2011;35:334-8. [DOI: 10.1192/pb.bp.110.033423] - DOI
Morrow 2012
Moscibrodzki 2018
-
- Moscibrodzki P, Katz CL.Application for inclusion to the 22nd expert committee on the selection and use of Essential Medicines: methylphenidate hydrochloride; 7 December 2018. Available at who.int/selection_medicines/committees/expert/22/applications/s24_methyl... (accessed 1 December 2021).
MTA 1999
Mühlbacher 2009
Mühlbacher 2010
Munkholm 2020
NICE 2008
-
- National Institute for Health and Care Excellence.Attention deficit hyperactivity disorder: diagnosis and management. Clinical guideline (CG72); 24 September 2008. Available at https://webarchive.nationalarchives.gov.uk/ukgwa/20140721215336/https://... (accessed 10 January 2021).
NICE 2018
-
- National Institute for Health and Care Excellence.Attention deficit hyperactivity disorder: diagnosis and management. NICE guideline (NG87); 14 March 2018. Available at www.nice.org.uk/guidance/ng87 (accessed 1 December 2021).
Page 2021
-
- Page M, Higgins JP, Sterne JA, editor(s).Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Paludan‐Müller 2021
Peterson 2008
Philipsen 2008
-
- Philipsen A.Comparison of a structured disorder tailored group psychotherapy plus placebo or methylphenidate versus psychiatric counseling plus placebo or methylphenidate in adult ADHD - a first randomised multicenter study [Vergleich einer strukturierten störungsspezifischen gruppenpsychotherapie plus placebo oder methylphenidat versus einer psychiatrischen beratung plus placebo oder methylphenidat bei ADHS im erwachsenenalter - eine erste randomisierte multizenter–studie (BMBF-ADHD-C1). Amendment 4. Protokollversion 5, 17.12.2008]. JAMA Psychiatry 2015;72(12):1190-210. [SUPPLEMENT 1: Protocol provided as an online supplement to JAMA Psychiatry] [WEBSITE: tinyurl.com/72ees8hq]
Philipsen 2014
-
- Philipsen A, Graf E, Jans T, Matthies S, Borel P, Colla M, et al.A randomized controlled multicenter trial on the multimodal treatment of adult attention-deficit hyperactivity disorder: enrollment and characteristics of the study sample. ADHD Attention Deficit and Hyperactivity Disorders 2014;6(1):35-47. [DOI: 10.1007/s12402-013-0120-z] [PMID: 24132867] - DOI - PubMed
Phillips 2014
Piper 2018
Pliszka 1989
Punja 2016
-
- Punja S, Shamseer L, Hartling L, Urichuk L, Vandermeer B, Nikles J, et al.Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database of Systematic Reviews 2016, Issue 2. Art. No: CD009996. [DOI: 10.1002/14651858.CD009996.pub2] [PMID: ] - DOI - PMC - PubMed
Raman 2018
-
- Raman SR, Man KK, Bahmanyar S, Berard A, Bilder S, Boukhris T, et al.Trends in attention-deficit hyperactivity disorder medication use: a retrospective observational study using population-based databases. Lancet Psychiatry 2018;5(10):824-35. [DOI: 10.1016/S2215-0366(18)30293-1] [PMID: ] - DOI - PubMed
Récalt 2019
Reimherr 2007
-
- Reimherr FW, Williams ED, Strong RE, Mestas R, Soni P, Merchant BK.A double-blind, placebo-controlled, crossover study of osmotic release oral system methylphenidate in adults with ADHD with assessment of oppositional and emotional dimensions of the disorder. Journal of Clinical Psychiatry 2007;68(1):93-101. [DOI: 10.4088/jcp.v68n0113] [PMID: ] - DOI - PubMed
Renoux 2016
-
- Renoux C, Shin JY, Dell'Aniello S, Fergusson E, Suissa S.Prescribing trends of attention‐deficit hyperactivity disorder (ADHD) medications in UK primary care, 1995–2015. British Journal of Clinical Pharmacology 2016;82(3):858-68. [DOI: 10.1111/bcp.13000] [PMCID: PMC5338115] [PMID: ] - DOI - PMC - PubMed
Review Manager 2020 [Computer program]
-
- Review Manager 5.4 (RevMan 5.4).Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Root 2019
-
- Root A, Brown JP, Forbes HJ, Bhaskaren K, Hayes J, Smeeth L, et al.Association of relative age in the school year with diagnosis of intellectual disability, attention-deficit/hyperactivity disorder, and depression. JAMA Pediatrics 2019;173(11):1068-75. [DOI: 10.1001/jamapediatrics.2019.3194] [PMCID: PMC6763997] [PMID: ] - DOI - PMC - PubMed
Safer 2016
Sayal 2017
Schatz 2015
Schelleman 2012
Schrantee 2016
-
- Schrantee A, Tamminga HG, Bouziane C, Bottelier MA, Bron EE, Mutsaerts HJ, et al.Age-dependent effects of methylphenidate on the human dopaminergic system in young vs adult patients with attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry 2016;73(9):955-62. [DOI: 10.1001/jamapsychiatry.2016.1572] [PMCID: PMC5267166] [PMID: ] - DOI - PMC - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s).Handbook for grading quality of evidence and strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shin 2016
-
- Shin JY, Roughead EE, Park BJ, Pratt NL.Cardiovascular safety of methylphenidate among children and young people with attention-deficit/hyperactivity disorder (ADHD): nationwide self controlled case series study. BMJ 2016;353:i2550. [DOI: 10.1136/bmj.i2550] [PMCID: PMC4887614] [PMID: ] - DOI - PMC - PubMed
Simon 2009
Sobanski 2007a
-
- Sobanski E, Brüggemann D, Alm B, Kern S, Deschner M, Schubert T, et al.Psychiatric comorbidity and functional impairment in a clinically referred sample of adults with attention-deficit/hyperactivity disorder (ADHD). European Archives of Psychiatry and Clinical Neuroscience 2007;257(7):371-7. [DOI: 10.1007/s00406-007-0712-8] [PMID: ] - DOI - PubMed
Sobanski 2007b
-
- Sobanski E, Alm B, Krumm B.Effect of subtype and psychiatric comorbidities on methylphenidate treatment in adults with attention-deficit hyperactivity disorder [Methylphenidatbehandlung bei Erwachsenen mit Aufmerksamkeitsdefizit-/Hyperaktivitätsstörung]. Der Nervenarzt 2007;78(3):328-30, 333-7. [DOI: 10.1007/s00115-006-2068-0] [PMID: ] - DOI - PubMed
Storebø 2015
-
- Storebø OJ, Ramstad E, Krogh HB, Nilausen TD, Skoog M, Holmskov M, et al.Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). Cochrane Database of Systematic Reviews 2015, Issue 11. Art. No: CD009885. [DOI: 10.1002/14651858.CD009885.pub2] [PMID: ] - DOI - PMC - PubMed
Storebø 2018
-
- Storebø OJ, Pedersen N, Ramstad E, Kielsholm ML, Nielsen SS, Krogh HB, et al.Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents – assessment of adverse events in non-randomised studies. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD012069. [DOI: 10.1002/14651858.CD012069.pub2] [PMCID: PMC6494554] [PMID: ] - DOI - PMC - PubMed
Storebø 2021
Sundhedsstyrelsen 2017
-
- Sundhedsstyrelsen [Danish Health Authority].Diagnostics and treatment of ADHD in adults. National clinical guideline; 2017 [Udredning og behandling af ADHD hos voksne. National Klinisk Retningslinje]. Available at sst.dk/-/media/Udgivelser/2015/NKR-ADHD-voksne/National-Klinisk-Retnings... (accessed 1 December 2021).
Surman 2010
-
- Surman CB, Monuteaux MC, Petty CR, Faraone SV, Spencer TJ, Chu NF, et al.Representativeness of participants in a clinical trial for attention-deficit/hyperactivity disorder? Comparison with adults from a large observational study. Journal of Clinical Psychiatry 2010;71(12):1612-6. [DOI: 10.4088/JCP.09m05344pur] [PMCID: PMC3737773] [PMID: ] - DOI - PMC - PubMed
Sussman 2006
Swanson 2017
-
- Swanson JM, Arnold LE, Molina BS, Sibley MH, Hechtman LT, Hinshaw SP, et al.Young adult outcomes in the follow-up of the multimodal treatment study of attention-deficit/hyperactivity disorder: symptom persistence, source discrepancy, and height suppression. Journal of Child Psycholology and Psychiatry 2017;58(6):663-78. [DOI: 10.1111/jcpp.12684] [PMID: ] - DOI - PMC - PubMed
Timimi 2004
Treceño 2012
-
- Treceño C, Martín Arias LH, Sáinz M, Salado I, García Ortega P, Velasco V, et al.Trends in the consumption of attention deficit hyperactivity disorder medications in Castilla y León (Spain): changes in the consumption pattern following the introduction of extended release methylphenidate. Pharmacoepidemiology and Drug Safety 2012;21(4):435-41. [DOI: 10.1002/pds.2348] [PMID: ] - DOI - PubMed
US DEA 2021
-
- US Department of Justice, Drug Enforcement Administration, Diversion Control Division.Controlled substance schedules. www.deadiversion.usdoj.gov/schedules/index.html (accessed 1 December 2021).
Wender 2011
WHO 1992
-
- World Health Organization.The ICD-10 Classification of Mental and Behavioural Disorders. Clinical Descriptions and Diagnostic Guidelines. Geneva (CH): World Health Organization, 1992. [URL: www.who.int/classifications/icd/en/bluebook.pdf]
WHO 2019a
-
- World Health Organization.ICD-11: International Classification of Diseases 11th Revision. icd.who.int/en (accessed 8 July 2020).
WHO 2019b
-
- World Health Organization.The selection and use of Essential Medicines: report of the WHO expert committee on selection and use of Essential Medicines, 2019 (including the 21st WHO Model List of Essential Medicines and the 7th WHO Model List of Essential Medicines for children); 2019. Available at apps.who.int/iris/bitstream/handle/10665/330668/9789241210300-eng.pdf?se... (accessed 1 December 2021).
WHO 2021a
-
- World Health Organization.Expert review 1. Expert Committee on Selection and Use of Essential Medicines. cdn.who.int/media/docs/default-source/essential-medicines/2021-eml-exper... (accessed 1 December 2021).
WHO 2021b
-
- World Health Organization.Expert review 2. Expert Committee on Selection and Use of Essential Medicines. cdn.who.int/media/docs/default-source/essential-medicines/2021-eml-exper... (accessed 1 December 2021).
Wilens 2008
-
- Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, et al.Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. Journal of the American Academy of Child and Adolescent Psychiatry 2008;47(1):21-31. [DOI: 10.1097/chi.0b013e31815a56f1] [PMID: ] - DOI - PubMed
References to other published versions of this review
Boesen 2017b
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

