Clinical Opportunities for Germline Pharmacogenetics and Management of Drug-Drug Interactions in Patients With Advanced Solid Cancers
- PMID: 35201852
- PMCID: PMC9848543
- DOI: 10.1200/PO.21.00312
Clinical Opportunities for Germline Pharmacogenetics and Management of Drug-Drug Interactions in Patients With Advanced Solid Cancers
Abstract
Purpose: Precision medicine approaches, including germline pharmacogenetics (PGx) and management of drug-drug interactions (DDIs), are likely to benefit patients with advanced cancer who are frequently prescribed multiple concomitant medications to treat cancer and associated conditions. Our objective was to assess the potential opportunities for PGx and DDI management within a cohort of adults with advanced cancer.
Methods: Medication data were collected from the electronic health records for 481 subjects since their first cancer diagnosis. All subjects were genotyped for variants with clinically actionable recommendations in Clinical Pharmacogenetics Implementation Consortium guidelines for 13 pharmacogenes. DDIs were defined as concomitant prescription of strong inhibitors or inducers with sensitive substrates of the same drug-metabolizing enzyme and were assessed for six major cytochrome P450 (CYP) enzymes.
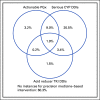
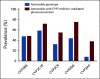
Results: Approximately 60% of subjects were prescribed at least one medication with Clinical Pharmacogenetics Implementation Consortium recommendations, and approximately 14% of subjects had an instance for actionable PGx, defined as a prescription for a drug in a subject with an actionable genotype. The overall subject-level prevalence of DDIs and serious DDIs were 50.3% and 34.8%, respectively. Serious DDIs were most common for CYP3A, CYP2D6, and CYP2C19, occurring in 24.9%, 16.8%, and 11.7% of subjects, respectively. When assessing PGx and DDIs together, approximately 40% of subjects had at least one opportunity for a precision medicine-based intervention and approximately 98% of subjects had an actionable phenotype for at least one CYP enzyme.
Conclusion: Our findings demonstrate numerous clinical opportunities for germline PGx and DDI management in adults with advanced cancer.
Conflict of interest statement
Figures
References
-
- Claassens DMF, Vos GJA, Bergmeijer TO, et al. : A genotype-guided strategy for oral P2Y(12) inhibitors in primary PCI. N Engl J Med 381:1621-1631, 2019 - PubMed
-
- Mallal S, Phillips E, Carosi G, et al. : HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med 358:568-579, 2008 - PubMed
-
- Pirmohamed M, Burnside G, Eriksson N, et al. : A randomized trial of genotype-guided dosing of warfarin. N Engl J Med 369:2294-2303, 2013 - PubMed
-
- Rosenblat JD, Lee Y, McIntyre RS: The effect of pharmacogenomic testing on response and remission rates in the acute treatment of major depressive disorder: A meta-analysis. J Affect Disord 241:484-491, 2018 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

