The Use of Off-Label Medications in Newborn Infants Despite an Approved Alternative Being Available-Results of a National Survey
- PMID: 35202069
- PMCID: PMC8877519
- DOI: 10.3390/pharmacy10010019
The Use of Off-Label Medications in Newborn Infants Despite an Approved Alternative Being Available-Results of a National Survey
Abstract
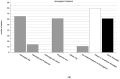
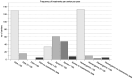
Neonates continue to be treated with off-label or unlicensed drugs while in hospital. However, some medications that have previously been used in adults underwent clinical testing and licensure for use with a different indication in the neonatal and pediatric population. Almost always, the marketing of these newly approved substances in a niche indication is accompanied by a steep increase in the price of the compound. We investigated the use of the approved formulation or the cheaper off-label alternative of Ibuprofen (Pedea®), Propanolol (Hemangiol®) and Caffeine Citrate (Peyona®) in neonatal clinical practice by conducting a National Survey of 214 Perinatal Centers in Germany. We also assessed price differences between on- and off-label alternatives and the extend of the clinical development program of the on-label medication in the neonatal population. On-label medication was more frequently used than the off-label alternative in all indications (PDA: on-label to off-label ratio 1:0.26, Apnea: 1:0.56, Hemangioma 1:0.76). All sponsors did conduct placebo-controlled Phase III trials with efficacy and safety endpoints in the target population and the number of participants in the target population varied between 82 and 497. Costs for the three drugs in their approved and marketed formulations increased in median 405-fold compared with the corresponding off-label alternative. Overall, about one out of three neonatologists prescribed an off-label or non-approved drug to patients despite an alternative medication that is approved for the indication in the target population being available.
Keywords: European pediatric regulation; NICU; neonate; off-label drug use; prescribing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

