Ultra-Long-Term Therapy of Benign Essential Blepharospasm with Botulinumtoxin A-30 Years of Experience in a Tertiary Care Center
- PMID: 35202148
- PMCID: PMC8875585
- DOI: 10.3390/toxins14020120
Ultra-Long-Term Therapy of Benign Essential Blepharospasm with Botulinumtoxin A-30 Years of Experience in a Tertiary Care Center
Abstract
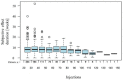
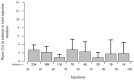
Aim of this study was to investigate the long-term results of botulinum toxin A (BoNT-A) injections for the treatment of benign essential blepharospasm (BEB) and to report our experience with (ultra-)long-term treatment with onabotulinumtoxin-A. We conducted a retrospective cross-sectional analysis at a university hospital. Patients with BEB and BoNT-A treatment were assigned to the Total Blepharospasm Group, patients with ≥21 onabotulinumtoxin-A injections to the Ona Long-Term Group. The Total Blepharospasm Group (n = 1940) included 33,933 BoNT-A injections. The age of patients at symptom onset was (mean ± SD) 58.0 ± 13.1 years, and 70.4% were female. The Ona long-term group (n = 234) included 10,632 onabotulinumtoxin-A injections. In this group, patients received 45.4 ± 22.9 injections with a mean dose of 22.2 IU ± 0.5. The duration of treatment was 12.6 ± 5.4 years, ranging from 2.9 to 30.0 years. The effect-duration-dose quotient did not change during long-term treatment. The observed side effects were comparable in type and frequency to other studies, even with the (ultra-)long treatment with onabotulinumtoxin-A. Our results, based on one of the largest patient populations and a treatment duration of up to 30 years, impressively demonstrate that onabotulinumtoxin-A is a safe and effective therapy for essential blepharospasm, even in the ultra-long term.
Keywords: adverse events; blepharospasm; botulinum toxin; long-term; outcome.
Conflict of interest statement
B.W. has received honoraria and travel grants from Merz and Desitin and research grants from Allergan and Merz. R.F. has no financial interests. P.R. received honoraria from Desitin. The sponsor had no influence on the choice of the research subject nor the design of the study, the representation or interpretation of the findings.
Figures



Similar articles
-
Long-term efficacy and safety of botulinum toxin A injections to treat blepharospasm and hemifacial spasm.Clin Exp Ophthalmol. 2014 Apr;42(3):254-61. doi: 10.1111/ceo.12165. Epub 2013 Aug 4. Clin Exp Ophthalmol. 2014. PMID: 23844601
-
Long-term Efficacy of Botulinum Neurotoxin-A Treatment for Essential Blepharospasm.Korean J Ophthalmol. 2018 Feb;32(1):1-7. doi: 10.3341/kjo.2017.0030. Epub 2018 Jan 24. Korean J Ophthalmol. 2018. PMID: 29376224 Free PMC article.
-
Long-term therapy of benign essential blepharospasm and facial hemispasm with botulinum toxin A: retrospective assessment of the clinical and quality of life impact in patients treated for more than 15 years.Acta Neurol Belg. 2014 Dec;114(4):285-91. doi: 10.1007/s13760-014-0285-z. Epub 2014 Mar 7. Acta Neurol Belg. 2014. PMID: 24604684
-
[Essential blepharospasm and hemifacial spasm: characteristic of the patient, botulinum toxin A treatment and literature review].Arq Bras Oftalmol. 2006 Jan-Feb;69(1):23-6. doi: 10.1590/s0004-27492006000100005. Epub 2006 Feb 10. Arq Bras Oftalmol. 2006. PMID: 16491229 Review. Portuguese.
-
Use of botulinum toxin in neuro-ophthalmology.Curr Opin Ophthalmol. 2001 Dec;12(6):419-22. doi: 10.1097/00055735-200112000-00006. Curr Opin Ophthalmol. 2001. PMID: 11734681 Review.
Cited by
-
Stable Convergent Polyneuronal Innervation and Altered Synapse Elimination in Orbicularis oculi Muscles from Patients with Blepharospasm Responding Poorly to Recurrent Botulinum Type-A Neurotoxin Injections.Toxins (Basel). 2024 Nov 24;16(12):506. doi: 10.3390/toxins16120506. Toxins (Basel). 2024. PMID: 39728764 Free PMC article.
-
Meta-Analysis of Adverse Reactions of Botulinum Toxin A in Facial Rejuvenation Treatment.Aesthetic Plast Surg. 2025 May;49(9):2305-2314. doi: 10.1007/s00266-024-04539-4. Epub 2024 Dec 2. Aesthetic Plast Surg. 2025. PMID: 39623228
-
Frequency of Hemorrhagic Side Effects of Botulinum Neurotoxin Treatment in Patients with Blepharospasm and Hemifacial Spasm on Antithrombotic Medication.Toxins (Basel). 2022 Nov 7;14(11):769. doi: 10.3390/toxins14110769. Toxins (Basel). 2022. PMID: 36356019 Free PMC article.
-
Botulinum Neurotoxin A Injections in Spasmodic Entropion: A Clinical Retrospective Cohort Study.Toxins (Basel). 2025 Jul 31;17(8):383. doi: 10.3390/toxins17080383. Toxins (Basel). 2025. PMID: 40864059 Free PMC article.
-
Depressive symptoms and quality of life in patients with benign essential blepharospasm under long-term therapy with botulinum toxin.Acta Neurol Belg. 2025 Feb;125(1):157-168. doi: 10.1007/s13760-024-02658-y. Epub 2024 Nov 1. Acta Neurol Belg. 2025. PMID: 39482421 Free PMC article.
References
-
- Roggenkämper P., Laskawi R. Botulinumtoxin-Therapie im Kopf-Hals-Bereich. Urban&Vogel; München, Germany: 2004.
-
- ESDE Epidemiological study of dystonia in Europe. J. Neurol. 2000;247:787–792. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

