Fungal Naphthalenones; Promising Metabolites for Drug Discovery: Structures, Biosynthesis, Sources, and Pharmacological Potential
- PMID: 35202181
- PMCID: PMC8879409
- DOI: 10.3390/toxins14020154
Fungal Naphthalenones; Promising Metabolites for Drug Discovery: Structures, Biosynthesis, Sources, and Pharmacological Potential
Abstract
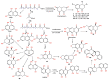
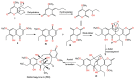
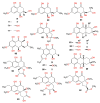
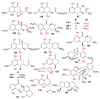
Fungi are well-known for their abundant supply of metabolites with unrivaled structure and promising bioactivities. Naphthalenones are among these fungal metabolites, that are biosynthesized through the 1,8-dihydroxy-naphthalene polyketide pathway. They revealed a wide spectrum of bioactivities, including phytotoxic, neuro-protective, cytotoxic, antiviral, nematocidal, antimycobacterial, antimalarial, antimicrobial, and anti-inflammatory. The current review emphasizes the reported naphthalenone derivatives produced by various fungal species, including their sources, structures, biosynthesis, and bioactivities in the period from 1972 to 2021. Overall, more than 167 references with 159 metabolites are listed.
Keywords: bioactivities; biosynthesis; fungi; naphthalenones; phytotoxic; polyketides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Secondary Metabolites from Acremonium Fungi: Diverse Structures and Bioactivities.Mini Rev Med Chem. 2017;17(7):603-632. doi: 10.2174/1389557516666160914194134. Mini Rev Med Chem. 2017. PMID: 27633747 Review.
-
Daldionin, an Unprecedented Binaphthyl Derivative, and Diverse Polyketide Congeners from a Fungal Orchid Endophyte.Chemistry. 2016 Mar 18;22(13):4551-5. doi: 10.1002/chem.201504005. Epub 2016 Feb 16. Chemistry. 2016. PMID: 26880363
-
An Updated Review of Secondary Metabolites from Marine Fungi.Mini Rev Med Chem. 2021;21(5):602-642. doi: 10.2174/1389557520666200925142514. Mini Rev Med Chem. 2021. PMID: 32981503 Review.
-
Bioactive deoxypreussomerins and dimeric naphthoquinones from Diospyros ehretioides fruits: deoxypreussomerins may not be plant metabolites but may be from fungal epiphytes or endophytes.Chem Biodivers. 2005 Oct;2(10):1358-67. doi: 10.1002/cbdv.200590108. Chem Biodivers. 2005. PMID: 17191937
-
Naturally Occurring Diterpenoid Dimers: Source, Biosynthesis, Chemistry and Bioactivities.Planta Med. 2016 Oct;82(15):1309-1328. doi: 10.1055/s-0042-114573. Epub 2016 Aug 19. Planta Med. 2016. PMID: 27542177 Review.
Cited by
-
Fungal Metabolomics: A Comprehensive Approach to Understanding Pathogenesis in Humans and Identifying Potential Therapeutics.J Fungi (Basel). 2025 Jan 24;11(2):93. doi: 10.3390/jof11020093. J Fungi (Basel). 2025. PMID: 39997385 Free PMC article. Review.
-
Genus Acanthella-A Wealthy Treasure: Secondary Metabolites, Synthesis, Biosynthesis, and Bioactivities.Mar Drugs. 2023 Apr 21;21(4):257. doi: 10.3390/md21040257. Mar Drugs. 2023. PMID: 37103397 Free PMC article. Review.
-
Docking and Molecular Dynamic Investigations of Phenylspirodrimanes as Cannabinoid Receptor-2 Agonists.Molecules. 2022 Dec 21;28(1):44. doi: 10.3390/molecules28010044. Molecules. 2022. PMID: 36615238 Free PMC article.
-
Electrochemical Dehydrogenative sp2-Coupling Reaction of Naphthols Accessing a Polycyclic Naphthalenone Motif.Org Lett. 2025 Jan 10;27(1):25-29. doi: 10.1021/acs.orglett.4c03518. Epub 2024 Dec 10. Org Lett. 2025. PMID: 39655743 Free PMC article.
-
Benzophenones-natural metabolites with great Hopes in drug discovery: structures, occurrence, bioactivities, and biosynthesis.RSC Adv. 2023 Aug 4;13(34):23472-23498. doi: 10.1039/d3ra02788k. eCollection 2023 Aug 4. RSC Adv. 2023. PMID: 37546221 Free PMC article. Review.
References
-
- Ibrahim S.R.M., Mohamed S.G.A., Sindi I.A., Mohamed G.A. Biologically active secondary metabolites and biotechnological applications of species of the family Chaetomiaceae (Sordariales): An updated review from 2016 to 2021. Mycol. Prog. 2021;20:595–639. doi: 10.1007/s11557-021-01704-w. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

