Predictive Risk Mapping of Schistosomiasis in Madagascar Using Ecological Niche Modeling and Precision Mapping
- PMID: 35202211
- PMCID: PMC8876685
- DOI: 10.3390/tropicalmed7020015
Predictive Risk Mapping of Schistosomiasis in Madagascar Using Ecological Niche Modeling and Precision Mapping
Abstract
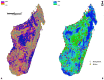
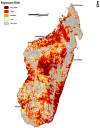
Schistosomiasis is a neglected tropical disease (NTD) found throughout tropical and subtropical Africa. In Madagascar, the condition is widespread and endemic in 74% of all administrative districts in the country. Despite the significant burden of the disease, high-resolution risk maps have yet to be produced to guide national control programs. This study used an ecological niche modeling (ENM) and precision mapping approach to estimate environmental suitability and disease transmission risk. The results show that suitability for schistosomiasis is widespread and covers 264,781 km2 (102,232 sq miles). Covariates of significance to the model were the accessibility to cities, distance to water, enhanced vegetation index (EVI), annual mean temperature, land surface temperature (LST), clay content, and annual precipitation. Disease transmission risk is greatest in the central highlands, tropical east coast, arid-southwest, and northwest. An estimated 14.9 million people could be at risk of schistosomiasis; 11.4 million reside in rural areas, while 3.5 million are in urban areas. This study provides valuable insight into the geography of schistosomiasis in Madagascar and its potential risk to human populations. Because of the focal nature of the disease, these maps can inform national surveillance programs while improving understanding of areas in need of medical interventions.
Keywords: disease mapping; ecological niche modeling; geographic information science; precision public health; schistosomiasis.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The findings and conclusions in this document are those of the author and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Figures



Similar articles
-
Mapping the Geographic Distribution of Tungiasis in Sub-Saharan Africa.Trop Med Infect Dis. 2020 Jul 24;5(3):122. doi: 10.3390/tropicalmed5030122. Trop Med Infect Dis. 2020. PMID: 32722011 Free PMC article.
-
Transmission Risk Predicting for Schistosomiasis in Mainland China by Exploring Ensemble Ecological Niche Modeling.Trop Med Infect Dis. 2022 Dec 28;8(1):24. doi: 10.3390/tropicalmed8010024. Trop Med Infect Dis. 2022. PMID: 36668931 Free PMC article.
-
Distribution of tungiasis in latin America: Identification of areas for potential disease transmission using an ecological niche model.Lancet Reg Health Am. 2021 Nov 1;5:100080. doi: 10.1016/j.lana.2021.100080. eCollection 2022 Jan. Lancet Reg Health Am. 2021. PMID: 36776459 Free PMC article.
-
[Impact of changes in the environment on vector-transmitted diseases].Sante. 1997 Jul-Aug;7(4):263-9. Sante. 1997. PMID: 9410453 Review. French.
-
[Malaria and schistosomiasis: 2 examples using systems of geographical information and teledetection in Madagascar].Bull Soc Pathol Exot. 2000 Jul;93(3):208-14. Bull Soc Pathol Exot. 2000. PMID: 11030060 Review. French.
Cited by
-
Modelling the historical distribution of schistosomiasis-transmitting snails in South Africa using ecological niche models.PLoS One. 2023 Nov 30;18(11):e0295149. doi: 10.1371/journal.pone.0295149. eCollection 2023. PLoS One. 2023. PMID: 38033142 Free PMC article.
-
Impact of environmental factors on Biomphalaria pfeifferi vector capacity leading to human infection by Schistosoma mansoni in two regions of western Côte d'Ivoire.Parasit Vectors. 2024 Apr 5;17(1):179. doi: 10.1186/s13071-024-06163-2. Parasit Vectors. 2024. PMID: 38581062 Free PMC article.
-
A potential transition from a concentrated to a generalized HIV epidemic: the case of Madagascar.Infect Dis Poverty. 2023 Dec 7;12(1):112. doi: 10.1186/s40249-023-01164-2. Infect Dis Poverty. 2023. PMID: 38057918 Free PMC article.
-
Ecological Niche Model of Bacillus cereus Group Isolates Containing a Homologue of the pXO1 Anthrax Toxin Genes Infecting Metalworkers in the United States.Pathogens. 2022 Apr 14;11(4):470. doi: 10.3390/pathogens11040470. Pathogens. 2022. PMID: 35456145 Free PMC article.
-
Open-source environmental data as an alternative to snail surveys to assess schistosomiasis risk in areas approaching elimination.Int J Health Geogr. 2023 Jun 2;22(1):12. doi: 10.1186/s12942-023-00331-w. Int J Health Geogr. 2023. PMID: 37268933 Free PMC article.
References
-
- Schistosomiasis. [(accessed on 11 November 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis.
-
- Livingston M., Pillay P., Zulu S.G., Sandvik L., Kvalsvig J.D., Gagai S., Galappaththi-Arachchige H.N., Kleppa E., Ndhlovu P., Vennervald B. Mapping Schistosoma Haematobium for Novel Interventions against Female Genital Schistosomiasis and Associated HIV Risk in KwaZulu-Natal, South Africa. Am. J. Trop. Med. Hyg. 2021;104:2055. doi: 10.4269/ajtmh.20-0679. - DOI - PMC - PubMed
-
- Pennance T., Allan F., Emery A., Rabone M., Cable J., Garba A.D., Hamidou A.A., Webster J.P., Rollinson D., Webster B.L. Interactions between Schistosoma Haematobium Group Species and Their Bulinus Spp. Intermediate Hosts along the Niger River Valley. Parasites Vectors. 2020;13:1–15. doi: 10.1186/s13071-020-04136-9. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

