Implementing a Substance-Use Screening and Intervention Program for People Living with Rifampicin-Resistant Tuberculosis: Pragmatic Experience from Khayelitsha, South Africa
- PMID: 35202216
- PMCID: PMC8879094
- DOI: 10.3390/tropicalmed7020021
Implementing a Substance-Use Screening and Intervention Program for People Living with Rifampicin-Resistant Tuberculosis: Pragmatic Experience from Khayelitsha, South Africa
Abstract
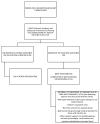
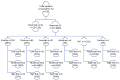
Substance use (SU) is associated with poor rifampicin-resistant tuberculosis (RR-TB) treatment outcomes. In 2017, a SBIRT (SU screening-brief intervention-referral to treatment) was integrated into routine RR-TB care in Khayelitsha, South Africa. This was a retrospective study of persons with RR-TB who were screened for SU between 1 July 2018 and 30 September 2020 using the ASSIST (Alcohol, Smoking and Substance Involvement Screening Test). Here we describe outcomes from this program. Persons scoring moderate/high risk received a brief intervention and referral to treatment. Overall, 333 persons were initiated on RR-TB treatment; 38% (n = 128) were screened for SU. Of those, 88% (n = 113/128) reported SU; 65% (n = 83/128) had moderate/high risk SU. Eighty percent (n = 103/128) reported alcohol use, of whom 52% (n = 54/103) reported moderate/high risk alcohol use. Seventy-seven persons were screened for SU within ≤2 months of RR-TB treatment initiation, of whom 69%, 12%, and 12% had outcomes of treatment success, loss to follow-up and death, respectively. Outcomes did not differ between persons with no/low risk and moderate/high risk SU or based on the receipt of naltrexone (p > 0.05). SU was common among persons with RR-TB; there is a need for interventions to address this co-morbidity as part of "person-centered care". Integrated, holistic care is needed at the community level to address unique challenges of persons with RR-TB and SU.
Keywords: ASSIST; RR-TB; SBIRT; brief intervention; integrated care; loss-to-follow-up; person-centered care; substance use.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- World Health Organization (WHO) Global Tuberculosis Report 2021. [(accessed on 1 November 2021)]. Available online: https://www.who.int/publications/i/item/9789240037021.
-
- Ahmad N., Ahuja S.D., Akkerman O.W., Alffenaar J.C., Anderson L.F., Baghaei P., Bang D., Barry P.M., Bastos M.L., Behera D., et al. Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB treatment–2017. Lancet. 2018;392:821–834. doi: 10.1016/S0140-6736(18)31644-1. - DOI - PMC - PubMed
-
- Shean K., Streicher E., Pieterson E., Symons G., van Zyl-Smit R., Theron G., Lehloenya R.J., Padanilam X., Wilcox P., Victor T.C., et al. Drug-Associated Adverse Events and Their Relationship with Outcomes in Patients Receiving Treatment for Extensively Drug-Resistant Tuberculosis in South Africa. PLoS ONE. 2013;8:e63057. doi: 10.1371/annotation/644591a8-8ae6-450e-974e-1cd1f08f52c7. - DOI - PMC - PubMed
-
- Morris M.D., Quezada L., Bhat P., Moser K., Smith J., Perez H., Laniado-Laborin R., Estrada-Guzman J., Rodwell T.C. Social, economic, and psychological impacts of MDR-TB treatment in Tijuana, Mexico: A patient’s perspective. Int. J. Tuberc. Lung Dis. 2013;17:954–960. doi: 10.5588/ijtld.12.0480. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

