Neuronal and Astrocytic Morphological Alterations Driven by Prolonged Exposure with Δ9-Tetrahydrocannabinol but Not Cannabidiol
- PMID: 35202235
- PMCID: PMC8879505
- DOI: 10.3390/toxics10020048
Neuronal and Astrocytic Morphological Alterations Driven by Prolonged Exposure with Δ9-Tetrahydrocannabinol but Not Cannabidiol
Abstract
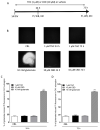
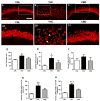
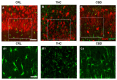
Cannabis derivatives are largely used in the general population for recreational and medical purposes, with the highest prevalence among adolescents, but chronic use and abuse has raised medical concerns. We investigated the prolonged effects of Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in organotypic hippocampal slices from P7 rats cultured for 2 weeks. Cell death in the CA1 subregion of slices was quantified by propidium iodide (PI) fluorescence, pre-synaptic and post-synaptic marker proteins were analysed by Western blotting and neurodegeneration and astrocytic alterations by NeuN and GFAP by immunofluorescence and confocal laser microscopy. The statistical significance of differences was analysed using ANOVA with a post hoc Dunnett w-test (PI fluorescence intensities and Western blots) or Newman-Keuls (immunohistochemistry data) for multiple comparisons. A probability value (P) of < 0.05 was considered significant. Prolonged (72 h) THC or CBD incubation did not induce cell death but caused modifications in the expression of synaptic proteins and morphological alterations in neurons and astrocytes. In particular, the expression of PSD95 was reduced following incubation for 72 h with THC and was increased following incubation with CBD. THC for 72 h caused disorganisation of CA1 stratum pyramidalis (SP) and complex morphological modifications in a significant number of pyramidal neurons and in astrocytes. Our results suggest that THC or CBD prolonged exposure induce different effects in the hippocampus. In particular, 72 h of THC exposure induced neuronal and glia alterations that must draw our attention to the effects that relatively prolonged use might cause, especially in adolescents.
Keywords: CBD; PSD95; THC; astrocyte; clasmatodendrosis; neuron; organotypic hippocampal slice; toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Zamberletti E., Gabaglio M., Grilli M., Prini P., Catanese A., Pittaluga A., Marchi M., Rubino T., Parolaro D. Long-term hippocampal glutamate synapse and astrocyte dysfunctions underlying the altered phenotype induced by adolescent THC treatment in male rats. Pharmacol. Res. 2016;111:459–470. doi: 10.1016/j.phrs.2016.07.008. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

