Effects of Zinc Oxide Nanoparticles on Model Systems of the Intestinal Barrier
- PMID: 35202236
- PMCID: PMC8880068
- DOI: 10.3390/toxics10020049
Effects of Zinc Oxide Nanoparticles on Model Systems of the Intestinal Barrier
Abstract
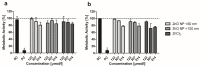
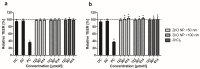
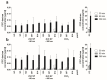
Zinc oxide nanoparticles (ZnO NP) are often used in the food sector, among others, because of their advantageous properties. As part of the human food chain, they are inevitably taken up orally. The debate on the toxicity of orally ingested ZnO NP continues due to incomplete data. Therefore, the aim of our study was to examine the effects of two differently sized ZnO NP (<50 nm and <100 nm primary particle size; 123-614 µmol/L) on two model systems of the intestinal barrier. Differentiated Caco-2 enterocytes were grown on Transwell inserts in monoculture and also in coculture with the mucus-producing goblet cell line HT29-MTX. Although no comprehensive mucus layer was detectable in the coculture, cellular zinc uptake was clearly lower after a 24-h treatment with ZnO NP than in monocultured cells. ZnO NP showed no influence on the permeability, metabolic activity, cytoskeleton and cell nuclei. The transepithelial electrical resistance was significantly increased in the coculture model after treatment with ≥307 µmol/L ZnO NP. Only small zinc amounts (0.07-0.65 µg/mL) reached the basolateral area. Our results reveal that the cells of an intact intestinal barrier interact with ZnO NP but do not suffer serious damage.
Keywords: Caco-2; HT29-MTX; Transwell; barrier integrity; coculture; nanoparticles; toxicity; zinc oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


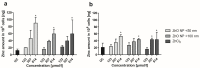
Similar articles
-
Impact of in vitro digested zinc oxide nanoparticles on intestinal model systems.Part Fibre Toxicol. 2022 May 30;19(1):39. doi: 10.1186/s12989-022-00479-6. Part Fibre Toxicol. 2022. PMID: 35644618 Free PMC article.
-
Cellular Uptake and Toxicological Effects of Differently Sized Zinc Oxide Nanoparticles in Intestinal Cells.Toxics. 2021 Apr 27;9(5):96. doi: 10.3390/toxics9050096. Toxics. 2021. PMID: 33925422 Free PMC article.
-
A tunable Caco-2/HT29-MTX co-culture model mimicking variable permeabilities of the human intestine obtained by an original seeding procedure.Eur J Pharm Biopharm. 2014 Jul;87(2):290-8. doi: 10.1016/j.ejpb.2014.03.017. Epub 2014 Apr 2. Eur J Pharm Biopharm. 2014. PMID: 24704198
-
Using 3D gastrointestinal tract in vitro models with microfold cells and mucus secreting ability to assess the hazard of copper oxide nanomaterials.J Nanobiotechnology. 2019 May 21;17(1):70. doi: 10.1186/s12951-019-0503-1. J Nanobiotechnology. 2019. PMID: 31113462 Free PMC article.
-
ZnO nanoparticles affect nutrient transport in an in vitro model of the small intestine.Food Chem Toxicol. 2019 Feb;124:112-127. doi: 10.1016/j.fct.2018.11.048. Epub 2018 Nov 29. Food Chem Toxicol. 2019. PMID: 30503572 Free PMC article.
Cited by
-
Nanoparticle Uptake and Crossing by Human In Vitro Models of Intestinal Barriers: A Scoping Review.Nanomaterials (Basel). 2025 Aug 5;15(15):1195. doi: 10.3390/nano15151195. Nanomaterials (Basel). 2025. PMID: 40801733 Free PMC article. Review.
-
An Adverse Outcome Pathway for food nanomaterial-induced intestinal barrier disruption.Front Toxicol. 2024 Dec 24;6:1474397. doi: 10.3389/ftox.2024.1474397. eCollection 2024. Front Toxicol. 2024. PMID: 39776762 Free PMC article.
-
Determinants and mechanisms of inorganic nanoparticle translocation across mammalian biological barriers.Arch Toxicol. 2023 Aug;97(8):2111-2131. doi: 10.1007/s00204-023-03528-x. Epub 2023 Jun 12. Arch Toxicol. 2023. PMID: 37303009 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

