Methodology and Neuromarkers for Cetaceans' Brains
- PMID: 35202291
- PMCID: PMC8879147
- DOI: 10.3390/vetsci9020038
Methodology and Neuromarkers for Cetaceans' Brains
Abstract
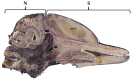
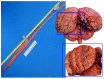
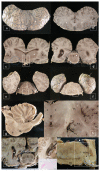
Cetacean brain sampling may be an arduous task due to the difficulty of collecting and histologically preparing such rare and large specimens. Thus, one of the main challenges of working with cetaceans' brains is to establish a valid methodology for an optimal manipulation and fixation of the brain tissue, which allows the samples to be viable for neuroanatomical and neuropathological studies. With this in view, we validated a methodology in order to preserve the quality of such large brains (neuroanatomy/neuropathology) and at the same time to obtain fresh brain samples for toxicological, virological, and microbiological analysis (neuropathology). A fixation protocol adapted to brains, of equal or even three times the size of human brains, was studied and tested. Finally, we investigated the usefulness of a panel of 20 antibodies (neuromarkers) associated with the normal structure and function of the brain, pathogens, age-related, and/or functional variations. The sampling protocol and some of the 20 neuromarkers have been thought to explore neurodegenerative diseases in these long-lived animals. To conclude, many of the typical measures used to evaluate neuropathological changes do not tell us if meaningful cellular changes have occurred. Having a wide panel of antibodies and histochemical techniques available allows for delving into the specific behavior of the neuronal population of the brain nuclei and to get a "fingerprint" of their real status.
Keywords: beaked whales; cetaceans; dolphins; immunohistochemistry; methodology; neuroanatomy; neuromarkers; neuropathology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Range-wide Yangtze freshwater dolphin expedition: The last chance to see Baiji?Environ Sci Pollut Res Int. 2006 Oct;13(6):418-24. doi: 10.1065/espr2006.10.350. Environ Sci Pollut Res Int. 2006. PMID: 17120833
-
Questioning the interpretations of behavioral observations of cetaceans: is there really support for a special intellectual status for this mammalian order?Neuroscience. 2013 Oct 10;250:664-96. doi: 10.1016/j.neuroscience.2013.07.041. Epub 2013 Jul 26. Neuroscience. 2013. PMID: 23896571 Review.
-
Cortical complexity in cetacean brains.Anat Rec A Discov Mol Cell Evol Biol. 2005 Nov;287(1):1142-52. doi: 10.1002/ar.a.20258. Anat Rec A Discov Mol Cell Evol Biol. 2005. PMID: 16200644 Review.
-
Skeletons of terrestrial cetaceans and the relationship of whales to artiodactyls.Nature. 2001 Sep 20;413(6853):277-81. doi: 10.1038/35095005. Nature. 2001. PMID: 11565023
-
Magnetic resonance images of the brain of a dwarf sperm whale (Kogia simus).J Anat. 2003 Jul;203(1):57-76. doi: 10.1046/j.1469-7580.2003.00199.x. J Anat. 2003. PMID: 12892406 Free PMC article.
Cited by
-
Structural brain preservation: a potential bridge to future medical technologies.Front Med Technol. 2024 Sep 9;6:1400615. doi: 10.3389/fmedt.2024.1400615. eCollection 2024. Front Med Technol. 2024. PMID: 39315362 Free PMC article.
-
The Hypothalamus of the Beaked Whales: The Paraventricular, Supraoptic, and Suprachiasmatic Nuclei.Biology (Basel). 2023 Oct 9;12(10):1319. doi: 10.3390/biology12101319. Biology (Basel). 2023. PMID: 37887029 Free PMC article.
-
Systematic validation and assessment of immunohistochemical markers for central nervous system pathology in cetaceans, with emphasis on auditory pathways.PLoS One. 2022 Jun 1;17(6):e0269090. doi: 10.1371/journal.pone.0269090. eCollection 2022. PLoS One. 2022. PMID: 35648776 Free PMC article.
-
Toothed Whales Have Black Neurons in the Blue Spot.Vet Sci. 2022 Sep 26;9(10):525. doi: 10.3390/vetsci9100525. Vet Sci. 2022. PMID: 36288139 Free PMC article.
-
Dolphin Pituitary Gland: Immunohistochemistry and Ultrastructural Cell Characterization Following a Novel Anatomical Dissection Protocol and Non-Invasive Imaging (MRI).Animals (Basel). 2025 Mar 4;15(5):735. doi: 10.3390/ani15050735. Animals (Basel). 2025. PMID: 40076018 Free PMC article.
References
-
- Cozzi B., Huggenberger S., Oelschläger H. Anatomy of Dolphins. Academic Press; San Diego, CA, USA: 2017. Chapter 3. Locomotion (Including Osteology and Myology) pp. 33–89.
-
- Huggenberger S. The size and complexity of dolphin brains—a paradox? J. Mar. Biol. Assoc. 2008;88:1103–1108. doi: 10.1017/S0025315408000738. - DOI
-
- Oelschläger H.H.A., Oelschläger J.S. Brain. In: Perrin W.F., Würsig B., Thewissen J.G.M., editors. Encyclopedia of Marine Mammals. 2nd ed. Academic Press; San Diego, CA, USA: 2002. pp. 134–149. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

