The Impact of Anticoagulation Agent on the Composition and Phenotype of Blood Leukocytes in Dromedary Camels
- PMID: 35202331
- PMCID: PMC8878879
- DOI: 10.3390/vetsci9020078
The Impact of Anticoagulation Agent on the Composition and Phenotype of Blood Leukocytes in Dromedary Camels
Abstract
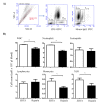
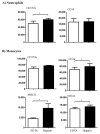
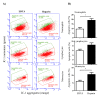
For the analysis of several cellular biomarkers, blood samples are anticoagulated using different agents with different modes of action. However, for the most commonly used anticoagulants, EDTA and heparin, varying effects on blood components have been reported in different species. As little is known about the impact of anticoagulants on the immunological evaluation of camel leukocytes, the present study analyzed the leukogram, the immunophenotype, and the cell vitality of camel leukocytes separated from blood samples anticoagulated with EDTA or lithium heparin. Using flow cytometry and staining with monoclonal antibodies to several cell surface markers, the composition and immunophenotype of camel leukocytes separated from blood anticoagulated with EDTA or heparin were analyzed. In comparison to EDTA-anticoagulated blood, using lithium heparin as an anticoagulant resulted in reduced numbers of total leukocytes and reduced numbers of neutrophils, which led to a reduced neutrophil to lymphocyte ratio. The analysis of cell necrosis and apoptosis after the staining of leukocytes with the DNA-sensitive dye propidium iodide and the mitochondrial membrane potential probe JC1 revealed a higher fraction of necrotic neutrophils and higher fractions of apoptotic neutrophils and monocytes in heparin blood than in EDTA blood. In addition, monocytes from heparin blood showed higher expression levels of the cell surface markers CD14, CD163, and MHCII when compared to cells from EDTA blood. Similarly, in heparin blood, CD44 and CD172a were expressed higher on neutrophils, while CD11a was expressed higher on lymphocytes in comparison to cells from EDTA blood. The results of the current study indicate the importance of considering the type of anticoagulant when investigating the composition, vitality, and immunophenotype of camel leukocytes.
Keywords: anticoagulant; cell biomarkers; dromedary camel; flow cytometry; immunophenotyping; leukocytes.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
Bacterial phagocytosis and reactive oxygen species production by camel neutrophils and monocytes are influenced by the type of anticoagulation agent.Vet World. 2021 Jul;14(7):1888-1893. doi: 10.14202/vetworld.2021.1888-1893. Epub 2021 Jul 24. Vet World. 2021. PMID: 34475713 Free PMC article.
-
Dromedary camel CD14high MHCIIhigh monocytes display inflammatory properties and are reduced in newborn camel calves.BMC Vet Res. 2020 Feb 18;16(1):62. doi: 10.1186/s12917-020-02285-8. BMC Vet Res. 2020. PMID: 32070351 Free PMC article.
-
Immunomodulatory Effects of the Cyclooxygenase Inhibitor Lornoxicam on Phenotype and Function of Camel Blood Leukocytes.Animals (Basel). 2021 Jul 6;11(7):2023. doi: 10.3390/ani11072023. Animals (Basel). 2021. PMID: 34359151 Free PMC article.
-
Immune cell composition of the bronchoalveolar lavage fluid in healthy and respiratory diseased dromedary camels.BMC Vet Res. 2022 Sep 21;18(1):353. doi: 10.1186/s12917-022-03446-7. BMC Vet Res. 2022. PMID: 36131278 Free PMC article.
-
[Biological properties and sensitivity to induction therapy of differentiated cells expressing atypical immunophenotype in acute leukemia of children].Folia Med Cracov. 2001;42(3):5-80. Folia Med Cracov. 2001. PMID: 12353422 Review. Polish.
Cited by
-
Human bone marrow stromal cells: the impact of anticoagulants on stem cell properties.Front Cell Dev Biol. 2023 Sep 18;11:1255823. doi: 10.3389/fcell.2023.1255823. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37791077 Free PMC article.
-
Milk Immune Cell Composition in Dromedary Camels With Subclinical Mastitis.Front Vet Sci. 2022 Apr 14;9:885523. doi: 10.3389/fvets.2022.885523. eCollection 2022. Front Vet Sci. 2022. PMID: 35498755 Free PMC article.
References
-
- Van Dongen J.J., Lhermitte L., Bottcher S., Almeida J., van der Velden V.H., Flores-Montero J., Rawstron A., Asnafi V., Lecrevisse Q., Lucio P., et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26:1908–1975. doi: 10.1038/leu.2012.120. - DOI - PMC - PubMed
-
- Farschtschi S., Mattes M., Hildebrandt A., Chiang D., Kirchner B., Kliem H., Pfaffl M.W. Development of an advanced flow cytometry based high-resolution immunophenotyping method to benchmark early immune response in dairy cows. Sci. Rep. 2021;11:22896. doi: 10.1038/s41598-021-02405-w. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

