FXS causing missense mutations disrupt FMRP granule formation, dynamics, and function
- PMID: 35202393
- PMCID: PMC8903291
- DOI: 10.1371/journal.pgen.1010084
FXS causing missense mutations disrupt FMRP granule formation, dynamics, and function
Abstract
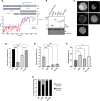
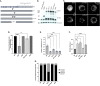
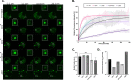
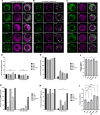
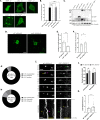
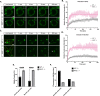
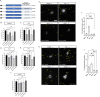
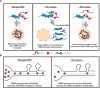
Fragile X Syndrome (FXS) is the most prevalent cause of inherited mental deficiency and is the most common monogenetic cause of autism spectral disorder (ASD). Here, we demonstrate that disease-causing missense mutations in the conserved K homology (KH) RNA binding domains (RBDs) of FMRP cause defects in its ability to form RNA transport granules in neurons. Using molecular, genetic, and imaging approaches in the Drosophila FXS model system, we show that the KH1 and KH2 domains of FMRP regulate distinct aspects of neuronal FMRP granule formation, dynamics, and transport. Furthermore, mutations in the KH domains disrupt translational repression in cells and the localization of known FMRP target mRNAs in neurons. These results suggest that the KH domains play an essential role in neuronal FMRP granule formation and function which may be linked to the molecular pathogenesis of FXS.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Fragile X Mental Retardation Protein positively regulates PKA anchor Rugose and PKA activity to control actin assembly in learning/memory circuitry.Neurobiol Dis. 2019 Jul;127:53-64. doi: 10.1016/j.nbd.2019.02.004. Epub 2019 Feb 13. Neurobiol Dis. 2019. PMID: 30771457 Free PMC article.
-
Conformational and dynamic properties of the KH1 domain of FMRP and its fragile X syndrome linked G266E variant.Biochim Biophys Acta Proteins Proteom. 2024 Jul 1;1872(4):141019. doi: 10.1016/j.bbapap.2024.141019. Epub 2024 Apr 17. Biochim Biophys Acta Proteins Proteom. 2024. PMID: 38641086
-
Molecular and cellular aspects of mental retardation in the Fragile X syndrome: from gene mutation/s to spine dysmorphogenesis.Adv Exp Med Biol. 2012;970:517-51. doi: 10.1007/978-3-7091-0932-8_23. Adv Exp Med Biol. 2012. PMID: 22351071 Review.
-
Neuron-Specific FMRP Roles in Experience-Dependent Remodeling of Olfactory Brain Innervation during an Early-Life Critical Period.J Neurosci. 2021 Feb 10;41(6):1218-1241. doi: 10.1523/JNEUROSCI.2167-20.2020. Epub 2021 Jan 5. J Neurosci. 2021. PMID: 33402421 Free PMC article.
-
The molecular biology of FMRP: new insights into fragile X syndrome.Nat Rev Neurosci. 2021 Apr;22(4):209-222. doi: 10.1038/s41583-021-00432-0. Epub 2021 Feb 19. Nat Rev Neurosci. 2021. PMID: 33608673 Free PMC article. Review.
Cited by
-
The feasibility and utility of hair follicle sampling to measure FMRP and FMR1 mRNA in children with or without fragile X syndrome: a pilot study.J Neurodev Disord. 2022 Dec 9;14(1):57. doi: 10.1186/s11689-022-09465-7. J Neurodev Disord. 2022. PMID: 36494616 Free PMC article.
-
Intracellular and intercellular transport of RNA organelles in CXG repeat disorders: The strength of weak ties.Front Mol Biosci. 2022 Dec 16;9:1000932. doi: 10.3389/fmolb.2022.1000932. eCollection 2022. Front Mol Biosci. 2022. PMID: 36589236 Free PMC article. Review.
-
Dysregulation of BMP, Wnt, and Insulin Signaling in Fragile X Syndrome.Front Cell Dev Biol. 2022 Jul 6;10:934662. doi: 10.3389/fcell.2022.934662. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35880195 Free PMC article. Review.
-
Folding Mechanism and Aggregation Propensity of the KH0 Domain of FMRP and Its R138Q Pathological Variant.Int J Mol Sci. 2022 Oct 12;23(20):12178. doi: 10.3390/ijms232012178. Int J Mol Sci. 2022. PMID: 36293035 Free PMC article.
-
FMRP cooperates with miRISC components to repress translation and regulate neurite morphogenesis in Drosophila.RNA Biol. 2024 Jan;21(1):11-22. doi: 10.1080/15476286.2024.2392304. Epub 2024 Aug 27. RNA Biol. 2024. PMID: 39190491 Free PMC article.
References
-
- Antar LN, Dictenberg JB, Plociniak M, Afroz R, Bassell GJ. Localization of FMRP-associated mRNA granules and requirement of microtubules for activity-dependent trafficking in hippocampal neurons. Genes Brain Behav. 2005;4(6):350–9. Epub 2005/08/16. doi: 10.1111/j.1601-183X.2005.00128.x . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

