Persistence of SARS-CoV-2 total immunoglobulins in a series of convalescent plasma and blood donors
- PMID: 35202394
- PMCID: PMC8870513
- DOI: 10.1371/journal.pone.0264124
Persistence of SARS-CoV-2 total immunoglobulins in a series of convalescent plasma and blood donors
Abstract
Background: The vast majority of COVID-19 cases both symptomatic and asymptomatic develop immunity after COVID-19 contagion. Whether lasting differences exist between infection and vaccination boosted immunity is yet to be known. The aim of this study was to determine how long total anti-SARS-CoV2 antibodies due to past infection persist in peripheral blood and whether sex, age or haematological features can influence their lasting.
Material and methods: A series of 2421 donations either of SARS-CoV-2 convalescent plasma or whole blood from 1107 repeat donors from January 2020 to March 2021 was analysed. An automated chemiluminescence immunoassay for total antibodies recognizing the nucleocapsid protein of SARS-CoV-2 in human serum and plasma was performed. Sex, age, blood group, blood cell counts and percentages and immunoglobulin concentrations were extracted from electronic recordings. Blood donation is allowed after a minimum of one-month post symptom's relapse. Donors were 69.7% males and their average age was 46. The 250 donors who had later donations after a positive one underwent further analysis. Both qualitative (positivity) and quantitative (rise or decline of optical density regarding consecutive donations) outcomes were evaluated.
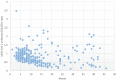
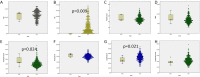
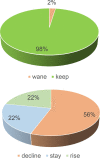
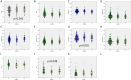
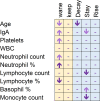
Results and discussion: In 97.6% of donors with follow-up, anti-SARS-CoV-2 protein N total antibodies remained positive at the end of a follow-up period of 12.4 weeks median time (1-46, SD = 9.65) after the first positive determination. The blood group was not related to antibody waning. Lower lymphocyte counts and higher neutrophils would help predict future waning or decay of antibodies. Most recovered donors maintain their total anti-SARS-CoV-2 N protein antibodies for at least 16 weeks (at least one month must have been awaited from infection resolution to blood donation). The 10 individuals that could be followed up longer than 40 weeks (approximately 44 weeks after symptom's relapse) were all still positive.
Conflict of interest statement
MCM participated as lecturer in two sponsored symposia managed by Roche. No other relevant relationship is to be declared relating to employment, consultancy, patents, products in development or marketed products. I hereby confirm this does not alter our adherence to PLOS ONE policies on sharing data and materials as detailed online in the guide for authors.
Figures

Similar articles
-
Blood group O convalescent plasma donations have significantly lower levels of SARS-CoV-2 IgG antibodies compared to blood group A donations.Transfusion. 2021 Aug;61(8):2245-2249. doi: 10.1111/trf.16524. Epub 2021 May 27. Transfusion. 2021. PMID: 34036595 Free PMC article.
-
Screening for SARS-CoV-2 antibodies in convalescent plasma in Brazil: Preliminary lessons from a voluntary convalescent donor program.Transfusion. 2020 Dec;60(12):2938-2951. doi: 10.1111/trf.16065. Epub 2020 Sep 16. Transfusion. 2020. PMID: 32935877 Free PMC article.
-
SARS-CoV-2 Antibody Avidity Responses in COVID-19 Patients and Convalescent Plasma Donors.J Infect Dis. 2020 Nov 13;222(12):1974-1984. doi: 10.1093/infdis/jiaa581. J Infect Dis. 2020. PMID: 32910175 Free PMC article.
-
Fresh frozen plasma for neutralizing SARS-CoV-2: "An exploratory cross-sectional study and review of the state of the art".Enferm Infecc Microbiol Clin (Engl Ed). 2025 May;43(5):282-290. doi: 10.1016/j.eimce.2025.03.014. Enferm Infecc Microbiol Clin (Engl Ed). 2025. PMID: 40340037 Review.
-
Antibody Responses in COVID-19: A Review.Front Immunol. 2021 Apr 15;12:633184. doi: 10.3389/fimmu.2021.633184. eCollection 2021. Front Immunol. 2021. PMID: 33936045 Free PMC article. Review.
Cited by
-
Assessment of the longitudinal humoral response in non-hospitalized SARS-CoV-2-positive individuals at decentralized sites: Outcomes and concordance.Front Immunol. 2023 Jan 20;13:1052424. doi: 10.3389/fimmu.2022.1052424. eCollection 2022. Front Immunol. 2023. PMID: 36741379 Free PMC article.
-
No indication for SARS-CoV-2 transmission to pet ferrets, in five cities in Poland, 2021 - antibody testing among ferrets living with owners infected with SARS-CoV-2 or free of infection.Acta Vet Scand. 2023 Feb 28;65(1):9. doi: 10.1186/s13028-023-00672-3. Acta Vet Scand. 2023. PMID: 36855124 Free PMC article.
-
Waning of anti-SARS-CoV-2 antibodies after the first wave of the COVID-19 pandemic in 2020: A 12-month-evaluation in three population-based European studies.PLoS One. 2025 May 9;20(5):e0320196. doi: 10.1371/journal.pone.0320196. eCollection 2025. PLoS One. 2025. PMID: 40344145 Free PMC article.
References
-
- Sekine T, Perez-Potti A, Rivera-Ballesteros O, Strålin K, Gorin JB, Olsson A, et al.. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell. 2020. Oct 1;183(1):158–168.e14. doi: 10.1016/j.cell.2020.08.017 Epub 2020 Aug 14. ; PMCID: PMC7427556. - DOI - PMC - PubMed
-
- Fenwick C, Croxatto A, Coste AT, Pojer F, André C, Pellaton C, et al.. Changes in SARS-CoV-2 Spike versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population-Based Seroprevalence Studies. J Virol. 2021. Jan 13;95(3):e01828–20. doi: 10.1128/JVI.01828-20 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

