Prediction of clinical trial enrollment rates
- PMID: 35202402
- PMCID: PMC8870517
- DOI: 10.1371/journal.pone.0263193
Prediction of clinical trial enrollment rates
Abstract
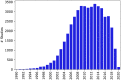
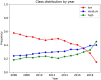
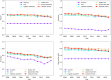
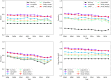
Clinical trials represent a critical milestone of translational and clinical sciences. However, poor recruitment to clinical trials has been a long standing problem affecting institutions all over the world. One way to reduce the cost incurred by insufficient enrollment is to minimize initiating trials that are most likely to fall short of their enrollment goal. Hence, the ability to predict which proposed trials will meet enrollment goals prior to the start of the trial is highly beneficial. In the current study, we leveraged a data set extracted from ClinicalTrials.gov that consists of 46,724 U.S. based clinical trials from 1990 to 2020. We constructed 4,636 candidate predictors based on data collected by ClinicalTrials.gov and external sources for enrollment rate prediction using various state-of-the-art machine learning methods. Taking advantage of a nested time series cross-validation design, our models resulted in good predictive performance that is generalizable to future data and stable over time. Moreover, information content analysis revealed the study design related features to be the most informative feature type regarding enrollment. Compared to the performance of models built with all features, the performance of models built with study design related features is only marginally worse (AUC = 0.78 ± 0.03 vs. AUC = 0.76 ± 0.02). The results presented can form the basis for data-driven decision support systems to assess whether proposed clinical trials would likely meet their enrollment goal.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Cheng SK, Dietrich MS, Dilts DM. A sense of urgency: evaluating the link between clinical trial development time and the accrual performance of cancer therapy evaluation program (NCI-CTEP) sponsored studies. Clinical Cancer Research. 2010;16(22):5557–5563. doi: 10.1158/1078-0432.CCR-10-0133 - DOI - PMC - PubMed
-
- Mendelsohn J, Moses HL, Nass SJ, et al. A national cancer clinical trials system for the 21st century: reinvigorating the NCI Cooperative Group Program. National Academies Press; 2010. - PubMed
-
- Unger JM, Vaidya R, Hershman DL, Minasian LM, Fleury ME. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. JNCI: Journal of the National Cancer Institute. 2019;111(3):245–255. doi: 10.1093/jnci/djy221 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

