Relationship of SARS-CoV-2 Antigen and Reverse Transcription PCR Positivity for Viral Cultures
- PMID: 35202532
- PMCID: PMC8888206
- DOI: 10.3201/eid2803.211747
Relationship of SARS-CoV-2 Antigen and Reverse Transcription PCR Positivity for Viral Cultures
Abstract
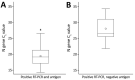
We assessed the relationship between antigen and reverse transcription PCR (RT-PCR) test positivity and successful virus isolation. We found that antigen test results were more predictive of virus recovery than RT-PCR results. However, virus was isolated from some antigen-negative and RT-PCR‒positive paired specimens, providing support for the Centers for Disease Control and Prevention antigen testing algorithm.
Keywords: COVID-19; SARS-CoV-2; coronavirus disease; coronaviruses; cycle threshold; rapid antigen test; respiratory infections; reverse transcription PCR; severe acute respiratory syndrome coronavirus 2; viral cultures; viruses; zoonoses.
Figures
References
-
- Prince-Guerra JL, Almendares O, Nolen LD, Gunn JKL, Dale AP, Buono SA, et al. Evaluation of Abbott BinaxNOW rapid antigen test for SARS-CoV-2 infection at two community-based testing sites—Pima County, Arizona, November 3–17, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:100–5. 10.15585/mmwr.mm7003e3 - DOI - PMC - PubMed
-
- Pollock NR, Jacobs JR, Tran K, Cranston AE, Smith S, O’Kane CY, et al. Performance and implementation evaluation of the Abbott BinaxNOW rapid antigen test in a high throughput drive-through community testing site in Massachusetts. J Clin Microbiol. 2021;59:e00083–21. 10.1128/JCM.00083-21 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

