T cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all individuals
- PMID: 35202566
- PMCID: PMC8810349
- DOI: 10.1016/j.cell.2022.01.029
T cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all individuals
Erratum in
-
T cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all individuals.Cell. 2022 Mar 31;185(7):1259. doi: 10.1016/j.cell.2022.03.022. Cell. 2022. PMID: 35364034 Free PMC article. No abstract available.
Abstract
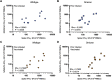
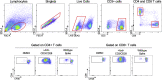
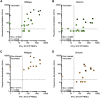
The SARS-CoV-2 Omicron variant (B.1.1.529) contains mutations that mediate escape from antibody responses, although the extent to which these substitutions in spike and non-spike proteins affect T cell recognition is unknown. In this study, we show that T cell responses in individuals with prior infection, vaccination, both prior infection and vaccination, and boosted vaccination are largely preserved to Omicron spike and non-spike proteins. However, we also identify a subset of individuals (∼21%) with a >50% reduction in T cell reactivity to the Omicron spike. Evaluation of functional CD4+ and CD8+ memory T cell responses confirmed these findings and revealed that reduced recognition to Omicron spike is primarily observed within the CD8+ T cell compartment potentially due to escape from HLA binding. Booster vaccination enhanced T cell responses to Omicron spike. In contrast to neutralizing immunity, these findings suggest preservation of T cell responses to the Omicron variant, although with reduced reactivity in some individuals.
Keywords: COVID-19; Delta; HLA; Omicron; SARS-CoV-2; T cell; epitopes; neutralization; vaccination; variants.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests G.D.G. has filed patent application PCT/US2021/028245.
Figures





Update of
-
T cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all prior infected and vaccinated individuals.medRxiv [Preprint]. 2022 Jan 5:2022.01.04.21268586. doi: 10.1101/2022.01.04.21268586. medRxiv. 2022. Update in: Cell. 2022 Mar 17;185(6):1041-1051.e6. doi: 10.1016/j.cell.2022.01.029. PMID: 35018386 Free PMC article. Updated. Preprint.
Comment in
-
T-cell immunity: a barrier to Omicron immune evasion.Signal Transduct Target Ther. 2022 Aug 28;7(1):297. doi: 10.1038/s41392-022-01142-4. Signal Transduct Target Ther. 2022. PMID: 36031636 Free PMC article. No abstract available.
References
-
- Barlos K., Chatzi O., Gatos D., Stavropoulos G. 2-Chlorotrityl chloride resin: studies on anchoring of Fmoc-amino acids and peptide cleavage. Int. J. Pept. Protein Res. 1991;37:513–520. - PubMed
-
- Garcia-Beltran W.F., St Denis K.J., Hoelzemer A., Lam E.C., Nitido A.D., Sheehan M.L., Berrios C., Ofoman O., Chang C.C., Hauser B.M., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. medRxiv. 2021 medRxiv:2021.12.14.21267755. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

