Regulation of GTPase function by autophosphorylation
- PMID: 35202574
- PMCID: PMC8986090
- DOI: 10.1016/j.molcel.2022.02.011
Regulation of GTPase function by autophosphorylation
Abstract
A unifying feature of the RAS superfamily is a conserved GTPase cycle by which these proteins transition between active and inactive states. We demonstrate that autophosphorylation of some GTPases is an intrinsic regulatory mechanism that reduces nucleotide hydrolysis and enhances nucleotide exchange, altering the on/off switch that forms the basis for their signaling functions. Using X-ray crystallography, nuclear magnetic resonance spectroscopy, binding assays, and molecular dynamics on autophosphorylated mutants of H-RAS and K-RAS, we show that phosphoryl transfer from GTP requires dynamic movement of the switch II region and that autophosphorylation promotes nucleotide exchange by opening the active site and extracting the stabilizing Mg2+. Finally, we demonstrate that autophosphorylated K-RAS exhibits altered effector interactions, including a reduced affinity for RAF proteins in mammalian cells. Thus, autophosphorylation leads to altered active site dynamics and effector interaction properties, creating a pool of GTPases that are functionally distinct from their non-phosphorylated counterparts.
Keywords: GTPase; NMR; RAF; RAS; RASSF; autophosphorylation; kinase; molecular dynamics; phosphoryl transfer; protein crystallography.
Copyright © 2022. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests K.M.H. is married to a member of Molecular Cell’s Advisory Board.
Figures
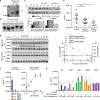




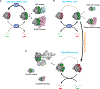
References
-
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, and Lindahl E. (2015). GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1-2, 19–25.
-
- Bergom C, Hauser AD, Rymaszewski A, Gonyo P, Prokop JW, Jennings BC, Lawton AJ, Frei A, Lorimer EL, Aguilera-Barrantes I, et al. (2016). The tumor-suppressive small GTPase DiRas1 binds the noncanonical guanine nucleotide exchange factor SmgGDS and antagonizes SmgGDS interactions with oncogenic small GTPases. J. Biol. Chem 291, 10948. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

