Expanding polysaccharide-protein coupling of glycoconjugate vaccines
- PMID: 35202656
- PMCID: PMC8914364
- DOI: 10.1016/j.jbc.2022.101755
Expanding polysaccharide-protein coupling of glycoconjugate vaccines
Abstract
For the preparation of glycoconjugate vaccines, polysaccharide antigens can usually be chemically modified to generate reactive functional groups (e.g., the formation of aldehyde groups by periodate oxidation of adjacent diols) for covalent coupling with proteins. In a recent issue of JBC, Duke et al. showed that an alternative agent, galactose oxidase (GOase) isolated from the fungus Fusarium sp. can generate aldehyde groups in a unique chemoenzymatic approach to prepare a conjugate vaccine against Streptococcus pneumoniae. These findings introduce a new strategy for the design and development of glycoconjugate vaccines.
Keywords: capsular polysaccharide; chemoenzymatic reaction; galactose oxidase; glycoconjugate vaccine.
Copyright © 2022 The Author. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest F. B. is an employee of the GSK group of companies. F. B. is listed as an inventor on patents owned by the GSK group of companies.
Figures
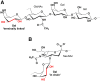
Comment on
-
Harnessing galactose oxidase in the development of a chemoenzymatic platform for glycoconjugate vaccine design.J Biol Chem. 2022 Jan;298(1):101453. doi: 10.1016/j.jbc.2021.101453. Epub 2021 Nov 25. J Biol Chem. 2022. PMID: 34838818 Free PMC article.
References
-
- Pizza M., Pecetta S., Rappuoli R. Vaccines 2020: The era of the digital vaccine is here. Sci. Transl. Med. 2021;13 - PubMed
-
- Berti F., Micoli F. Improving efficacy of glycoconjugate vaccines: From chemical conjugates to next generation constructs. Curr. Opin. Immunol. 2020;65:42–49. - PubMed
-
- Costantino P., Rappuoli R., Berti F. The design of semi-synthetic and synthetic glycoconjugate vaccines. Expert Opin. Drug Discov. 2011;6:1045–1066. - PubMed
-
- Anderson P.W., Pichichero M.E., Insel R.A., Betts R., Eby R., Smith D.H. Vaccines consisting of periodate-cleaved oligosaccharides from the capsule of Haemophilus influenzae type b coupled to a protein carrier: Structural and temporal requirements for priming in the human infant. J. Immunol. 1986;137:1181–1186. - PubMed
-
- Zou W., Jennings H.J. In: Carbohydrate-Based Vaccines and Immunotherapies. Guo Z., Boons G.-J., editors. John Wiley & Sons, Inc; Hoboken, NJ: 2009. Preparation of glycoconjugate vaccines; pp. 55–88.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

