Diminishing Immune Responses against Variants of Concern in Dialysis Patients 4 Months after SARS-CoV-2 mRNA Vaccination
- PMID: 35203113
- PMCID: PMC8962909
- DOI: 10.3201/eid2804.211907
Diminishing Immune Responses against Variants of Concern in Dialysis Patients 4 Months after SARS-CoV-2 mRNA Vaccination
Abstract
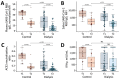
Patients undergoing chronic hemodialysis were among the first to receive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccinations because of their increased risk for severe coronavirus disease and high case-fatality rates. By using a previously reported cohort from Germany of at-risk hemodialysis patients and healthy donors, where antibody responses were examined 3 weeks after the second vaccination, we assessed systemic cellular and humoral immune responses in serum and saliva 4 months after vaccination with the Pfizer-BioNTech BNT162b2 vaccine using an interferon-γ release assay and multiplex-based IgG measurements. We further compared neutralization capacity of vaccination-induced IgG against 4 SARS-CoV-2 variants of concern (Alpha, Beta, Gamma, and Delta) by angiotensin-converting enzyme 2 receptor-binding domain competition assay. Sixteen weeks after second vaccination, compared with 3 weeks after, cellular and humoral responses against the original SARS-CoV-2 isolate and variants of concern were substantially reduced. Some dialysis patients even had no detectable B- or T-cell responses.
Keywords: COVID-19; SARS-CoV-2; booster dose; coronavirus disease; dialysis; immunocompromised; longevity of immune response; mRNA vaccination; respiratory infections; severe acute respiratory syndrome coronavirus 2; vaccine-preventable diseases; variants of concern; viruses; zoonoses.
Figures
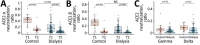
References
-
- European Centre for Disease Control and Prevention. Overview of the implementation of COVID-19 vaccination strategies and deployment plans in the EU/EEA. 2021. [cited 2021 Aug 18]. https://www.ecdc.europa.eu/en/publications-data/overview-implementation-...
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

