Calcineurin Controls Cellular Prion Protein Expression in Mouse Astrocytes
- PMID: 35203261
- PMCID: PMC8870693
- DOI: 10.3390/cells11040609
Calcineurin Controls Cellular Prion Protein Expression in Mouse Astrocytes
Abstract
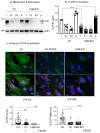
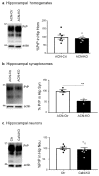
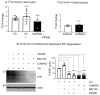
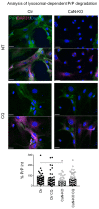
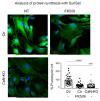
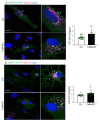
Prion diseases arise from the conformational conversion of the cellular prion protein (PrPC) into a self-replicating prion isoform (PrPSc). Although this process has been studied mostly in neurons, a growing body of evidence suggests that astrocytes express PrPC and are able to replicate and accumulate PrPSc. Currently, prion diseases remain incurable, while downregulation of PrPC represents the most promising therapy due to the reduction of the substrate for prion conversion. Here we show that the astrocyte-specific genetic ablation or pharmacological inhibition of the calcium-activated phosphatase calcineurin (CaN) reduces PrPC expression in astrocytes. Immunocytochemical analysis of cultured CaN-KO astrocytes and isolation of synaptosomal compartments from the hippocampi of astrocyte-specific CaN-KO (ACN-KO) mice suggest that PrPC is downregulated both in vitro and in vivo. The downregulation occurs without affecting the glycosylation of PrPC and without alteration of its proteasomal or lysosomal degradation. Direct assessment of the protein synthesis rate and shotgun mass spectrometry proteomics analysis suggest that the reduction of PrPC is related to the impairment of global protein synthesis in CaN-KO astrocytes. When WT-PrP and PrP-D177N, a mouse homologue of a human mutation associated with the inherited prion disease fatal familial insomnia, were expressed in astrocytes, CaN-KO astrocytes showed an aberrant localization of both WT-PrP and PrP-D177N variants with predominant localization to the Golgi apparatus, suggesting that ablation of CaN affects both WT and mutant PrP proteins. These results provide new mechanistic details in relation to the regulation of PrP expression in astrocytes, suggesting the therapeutic potential of astroglial cells.
Keywords: FK506; astrocytes; calcineurin; calcium; neuroinflammation; prion protein; protein synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Takada L.T., Kim M.-O., Metcalf S., Gala I.I., Geschwind M.D. Chapter 29—Prion Disease. In: Geschwind D.H., Paulson H.L., Klein C., editors. Handbook of Clinical Neurology. Volume 148. Elsevier; Amsterdam, The Netherlands: 2018. pp. 441–464. Neurogenetics, Part II. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

