Chromatoid Bodies in the Regulation of Spermatogenesis: Novel Role of GRTH
- PMID: 35203264
- PMCID: PMC8870266
- DOI: 10.3390/cells11040613
Chromatoid Bodies in the Regulation of Spermatogenesis: Novel Role of GRTH
Abstract
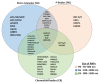
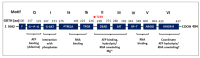
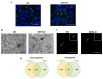
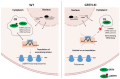
Post-transcriptional and translational control of specialized genes play a critical role in the progression of spermatogenesis. During the early stages, mRNAs are actively transcribed and stored, temporarily bound to RNA binding proteins in chromatoid bodies (CBs). CBs are membrane-less dynamic organelles which serve as storehouses and processing centers of mRNAs awaiting translation during later stages of spermatogenesis. These CBs can also regulate the stability of mRNAs to secure the correct timing of protein expression at different stages of sperm formation. Gonadotropin-regulated testicular RNA helicase (GRTH/DDX25) is an essential regulator of spermatogenesis. GRTH transports mRNAs from the nucleus to the cytoplasm and phospho-GRTH transports mRNAs from the cytoplasm to the CBs. During spermiogenesis, there is precise control of mRNAs transported by GRTH from and to the CBs, directing the timing of translation of critical proteins which are involved in spermatid elongation and acrosomal development, resulting in functional sperm formation. This chapter presents our current knowledge on the role of GRTH, phospho-GRTH and CBs in the control of spermiogenesis. In addition, it covers the components of CBs compared to those of stress granules and P-bodies.
Keywords: RNA storage; RNA transport; chromatoid bodies; phospho-GRTH; spermatogenesis; transcriptome analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kavarthapu R., Anbazhagan R., Raju M., Morris C.T., Pickel J., Dufau M.L. Targeted knock-in mice with a human mutation in GRTH/DDX25 reveals the essential role of phosphorylated GRTH in spermatid development during spermatogenesis. Hum. Mol. Genet. 2019;28:2561–2572. doi: 10.1093/hmg/ddz079. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

