Mitochondrial Neurodegeneration
- PMID: 35203288
- PMCID: PMC8870525
- DOI: 10.3390/cells11040637
Mitochondrial Neurodegeneration
Abstract
Mitochondria are cytoplasmic organelles, which generate energy as heat and ATP, the universal energy currency of the cell. This process is carried out by coupling electron stripping through oxidation of nutrient substrates with the formation of a proton-based electrochemical gradient across the inner mitochondrial membrane. Controlled dissipation of the gradient can lead to production of heat as well as ATP, via ADP phosphorylation. This process is known as oxidative phosphorylation, and is carried out by four multiheteromeric complexes (from I to IV) of the mitochondrial respiratory chain, carrying out the electron flow whose energy is stored as a proton-based electrochemical gradient. This gradient sustains a second reaction, operated by the mitochondrial ATP synthase, or complex V, which condensates ADP and Pi into ATP. Four complexes (CI, CIII, CIV, and CV) are composed of proteins encoded by genes present in two separate compartments: the nuclear genome and a small circular DNA found in mitochondria themselves, and are termed mitochondrial DNA (mtDNA). Mutations striking either genome can lead to mitochondrial impairment, determining infantile, childhood or adult neurodegeneration. Mitochondrial disorders are complex neurological syndromes, and are often part of a multisystem disorder. In this paper, we divide the diseases into those caused by mtDNA defects and those that are due to mutations involving nuclear genes; from a clinical point of view, we discuss pediatric disorders in comparison to juvenile or adult-onset conditions. The complementary genetic contributions controlling organellar function and the complexity of the biochemical pathways present in the mitochondria justify the extreme genetic and phenotypic heterogeneity of this new area of inborn errors of metabolism known as 'mitochondrial medicine'.
Keywords: Leigh syndrome; MELAS; MERRF; OXPHOS; POLG; mitochondrial disease; mitochondrial respiratory chain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

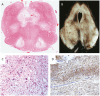
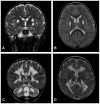
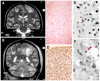

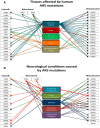
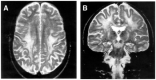
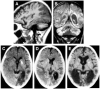
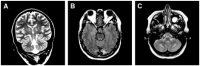
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

