Epigenetic Regulation of Cellular Senescence
- PMID: 35203320
- PMCID: PMC8870565
- DOI: 10.3390/cells11040672
Epigenetic Regulation of Cellular Senescence
Abstract
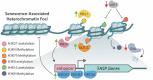
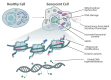
Senescence is a complex cellular stress response that abolishes proliferative capacity and generates a unique secretory pattern that is implicated in organismal aging and age-related disease. How a cell transitions to a senescent state is multifactorial and often requires transcriptional regulation of multiple genes. Epigenetic alterations to DNA and chromatin are powerful regulators of genome architecture and gene expression, and they play a crucial role in mediating the induction and maintenance of senescence. This review will highlight the changes in chromatin, DNA methylation, and histone alterations that establish and maintain cellular senescence, alongside the specific epigenetic regulation of the senescence-associated secretory phenotype (SASP).
Keywords: DNA methylation; SASP; aging; epigenetics; histone modification; senescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Demaria M., Ohtani N., Youssef S.A., Rodier F., Toussaint W., Mitchell J.R., Laberge R.-M., Vijg J., Van Steeg H., Dollé M.E., et al. An Essential Role for Senescent Cells in Optimal Wound Healing through Secretion of PDGF-AA. Dev. Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

