L-Arginine Depletion Improves Spinal Cord Injury via Immunomodulation and Nitric Oxide Reduction
- PMID: 35203413
- PMCID: PMC8869469
- DOI: 10.3390/biomedicines10020205
L-Arginine Depletion Improves Spinal Cord Injury via Immunomodulation and Nitric Oxide Reduction
Abstract
Background: Spinal cord injury (SCI) elicits robust neuroinflammation that eventually exacerbates the initial damage to the spinal cord. L-arginine is critical for the responsiveness of T cells, which are important contributors to neuroinflammation after SCI. Furthermore, L-arginine is the substrate for nitric oxide (NO) production, which is a known inducer of secondary damage.
Methods: To accomplish systemic L-arginine depletion, repetitive injections of recombinant arginase-1 (rArg-I) were performed. Functional recovery and histopathological parameters were analyzed. Splenic immune responses were evaluated by flow cytometry. Pro-inflammatory gene expression and nitrite concentrations were measured.
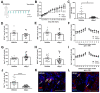
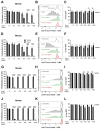
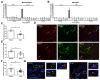
Results: We show for the first time that systemic L-arginine depletion improves locomotor recovery. Flow cytometry and immunohistological analysis showed that intraspinal T-cell infiltration was reduced by 65%, and peripheral numbers of Th1 and Th17 cells were suppressed. Moreover, rArg-I treatment reduced the intraspinal NO production by 40%. Histopathological analyses revealed a 37% and 36% decrease in the number of apoptotic neurons and neuron-macrophage/microglia contacts in the spinal cord, respectively.
Conclusions: Targeting detrimental T-cell responses and NO-production via rArg-I led to a reduced neuronal cell death and an improved functional recovery. These findings indicate that L-arginine depletion holds promise as a therapeutic strategy after SCI.
Keywords: CNS trauma; T cells; arginase-1; neuroinflammation; nitric oxide.
Conflict of interest statement
Hendrix is the founder of smartsciencecareer.com, which does not affect the data presented here. All co-authors of Bio-Cancer treatment International have no conflicts of interest in the present study for publication. P.C. is the founder of Biocancer Treatment International Ltd. P.C., G.S. and S.B. are listed as inventors on a patent (US9789169B2) covering recArg1/BCT-100. The remaining authors declare no competing interests.
Figures


References
-
- Beck K.D., Nguyen H.X., Galvan M.D., Salazar D.L., Woodruff T.M., Anderson A.J. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: Evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133 (Pt. 2):433–447. doi: 10.1093/brain/awp322. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

