VEGF-Trap Modulates Retinal Inflammation in the Murine Oxygen-Induced Retinopathy (OIR) Model
- PMID: 35203414
- PMCID: PMC8869660
- DOI: 10.3390/biomedicines10020201
VEGF-Trap Modulates Retinal Inflammation in the Murine Oxygen-Induced Retinopathy (OIR) Model
Abstract
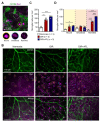
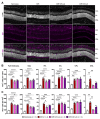
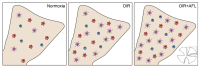
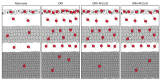
Anti-Vascular Endothelial Growth Factor (VEGF) agents are the first-line treatment for retinal neovascular diseases, which represent the most prevalent causes of acquired vision loss world-wide. VEGF-Trap (Aflibercept, AFL), a recombinant decoy receptor recognizing ligands of both VEGFR-1 and -2, was recently reported to be highly efficient in improving visual acuity and preserving retinal anatomy in individuals affected by diabetic macular edema. However, the precise molecular and cell biological mechanisms underlying the beneficial effects of this novel tool have yet to be elucidated. Using the mouse oxygen-induced retinopathy (OIR) model as a surrogate of retinopathies with sterile post-ischemic inflammation, such as late proliferative diabetic retinopathy (PDR), retinopathy of prematurity (ROP), and diabetic macular edema (DME), we provide evidence that AFL modulates inflammation in response to hypoxia by regulating the morphology of microglial cells, a parameter commonly used as a proxy for changes in their activation state. We show that AFL administration during the hypoxic period of OIR leads to an increased number of ramified Iba1+ microglial cells/macrophages while subsequently limiting the accumulation of these cells in particular retinal layers. Our results suggest that, beyond its well-documented beneficial effects on microvascular regeneration, AFL might exert important modulatory effects on post-ischemic retinal inflammation.
Keywords: Aflibercept; OIR; VEGF-Trap; inflammation; microglia; oxygen-induced retinopathy; retina.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

