Pd2Spermine Complex Shows Cancer Selectivity and Efficacy to Inhibit Growth of Triple-Negative Breast Tumors in Mice
- PMID: 35203420
- PMCID: PMC8869242
- DOI: 10.3390/biomedicines10020210
Pd2Spermine Complex Shows Cancer Selectivity and Efficacy to Inhibit Growth of Triple-Negative Breast Tumors in Mice
Abstract
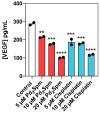
Pd2Spm is a dinuclear palladium(II)-spermine chelate with promising anticancer properties against triple-negative breast cancer (TNBC), a breast carcinoma subset with poor prognosis and limited treatment options. The present study evaluated the in vitro and in vivo anticancer effects of Pd2Spm compared to the reference metal-based drug cisplatin. Triple-negative breast cancer MDA-MB-231 cells, non-cancerous MCF-12A breast cells and chorioallantoic membrane (CAM) assay were used for antiproliferative, antimigratory and antiangiogenic studies. For an in vivo efficacy study, female CBA nude mice with subcutaneously implanted MDA-MB-231 breast tumors were treated with Pd2Spm (5 mg/kg/day) or cisplatin (2 mg/kg/day) administered intraperitoneally during 5 consecutive days. Promising selective antiproliferative activity of Pd2Spm was observed in MDA-MB-231 cells (IC50 values of 7.3-8.3 µM), with at least 10-fold lower activity in MCF-12A cells (IC50 values of 89.5-228.9 µM). Pd2Spm inhibited the migration of MDA-MB-231 cells, suppressed angiogenesis in CAM and decreased VEGF secretion from MDA-MB-231 cells with similar potency as cisplatin. Pd2Spm-treated mice showed a significant reduction in tumor growth progression, and tumors evidenced a reduction in the Ki-67 proliferation index and number of mitotic figures, as well as increased DNA damage, similar to cisplatin-treated animals. Encouragingly, systemic toxicity (hematotoxicity and weight loss) observed in cisplatin-treated animals was not observed in Pd2Spm-treated mice. The present study reports, for the first time, promising cancer selectivity, in vivo antitumor activity towards TNBC and a low systemic toxicity of Pd2Spm. Thus, this agent may be viewed as a promising Pd(II) drug candidate for the treatment of this type of low-prognosis neoplasia.
Keywords: Pd(II)-based drugs; cisplatin; in vivo; metal complexes; triple-negative breast cancer; xenografts.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

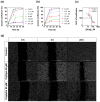

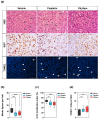
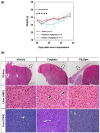

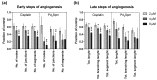
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

