Expression of the Adenosine A2A-A3 Receptor Heteromer in Different Brain Regions and Marked Upregulation in the Microglia of the Transgenic APPSw,Ind Alzheimer's Disease Model
- PMID: 35203424
- PMCID: PMC8869194
- DOI: 10.3390/biomedicines10020214
Expression of the Adenosine A2A-A3 Receptor Heteromer in Different Brain Regions and Marked Upregulation in the Microglia of the Transgenic APPSw,Ind Alzheimer's Disease Model
Abstract
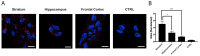
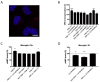
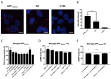
Adenosine (Ado) receptors have been instrumental in the detection of heteromers and other higher-order receptor structures, mainly via interactions with other cell surface G-protein-coupled receptors. Apart from the first report of the A1 Ado receptor interacting with the A2A Ado receptor, there has been more recent data on the possibility that every Ado receptor type, A1, A2A, A2B, and A3, may interact with each other. The aim of this paper was to look for the expression and function of the A2A/A3 receptor heteromer (A2AA3Het) in neurons and microglia. In situ proximity ligation assays (PLA), performed in primary cells, showed that A2AA3Het expression was markedly higher in striatal than in cortical and hippocampal neurons, whereas it was similar in resting and activated microglia. Signaling assays demonstrated that the effect of the A2AR agonist, PSB 777, was reduced in the presence of the A3R agonist, 2-Cl-IB-MECA, whereas the effect of the A3R agonist was potentiated by the A2AR antagonist, SCH 58261. Interestingly, the expression of the heteromer was markedly enhanced in microglia from the APPSw,Ind model of Alzheimer's disease. The functionality of the heteromer in primary microglia from APPSw,Ind mice was more similar to that found in resting microglia from control mice.
Keywords: G protein-coupled receptor; activated microglia; cerebral cortex; dementia; hippocampus; neurodegenerative disease; neuroinflammation; purinergic signaling; striatum.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Gines S., Hillion J., Torvinen M., Le Crom S., Casado V., Canela E.I., Rondin S., Lew J.Y., Watson S., Zoli M., et al. Dopamine D1 and Adenosine A1 Receptors form Functionally Interacting Heteromeric Complexes. Proc. Natl. Acad. Sci. USA. 2000;97:8606–8611. doi: 10.1073/pnas.150241097. - DOI - PMC - PubMed
-
- Hillion J., Canals M., Torvinen M., Casado V., Scott R., Terasmaa A., Hansson A., Watson S., Olah M.E., Mallol J., et al. Coaggregation, Cointernalization, and Codesensitization of Adenosine A2A Receptors and Dopamine D2 Receptors. [(accessed on 21 March 2015)];J. Biol. Chem. 2002 277:18091–18097. doi: 10.1074/jbc.M107731200. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11872740. - DOI - PubMed
-
- Ciruela F., Casadó V., Rodrigues R., Luján R., Burgueño J., Canals M., Borycz J., Rebola N., Goldberg S., Mallol J., et al. Presynaptic Control of Striatal Glutamatergic Neurotransmission by Adenosine A1-A2A Receptor Heteromers. J. Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. - DOI - PMC - PubMed
-
- Navarro G., Cordomí A., Brugarolas M., Moreno E., Aguinaga D., Pérez-Benito L., Ferre S., Cortés A., Casadó V., Mallol J., et al. Cross-Communication between Gi and Gs in a G-Protein-Coupled Receptor Heterotetramer Guided by a Receptor C-Terminal Domain. BMC Biol. 2018;16:1–15. doi: 10.1186/s12915-018-0491-x. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

